In vitro and in vivo pharmacological evaluation of the synthetic cannabinoid receptor agonist EG-018
- PMID: 32247816
- PMCID: PMC7239729
- DOI: 10.1016/j.pbb.2020.172918
In vitro and in vivo pharmacological evaluation of the synthetic cannabinoid receptor agonist EG-018
Abstract
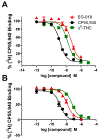
Synthetic cannabinoid receptor agonists (SCRAs) possess high abuse liability and complex toxicological profiles, making them serious threats to public health. EG-018 is a SCRA that has been detected in both illicit products and human samples, but it has received little attention to date. The current studies investigated EG-018 at human CB1 and CB2 receptors expressed in HEK293 cells in [3H]CP55,940 competition binding, [35S]GTPγS binding and forskolin-stimulated cAMP production. EG-018 was also tested in vivo for its ability to produce cannabimimetic and abuse-related effects in the cannabinoid tetrad and THC drug discrimination, respectively. EG-018 exhibited high affinity at CB1 (21 nM) and at CB2 (7 nM), but in contrast to typical SCRAs, behaved as a weak partial agonist in [35S]GTPγS binding, exhibiting lower efficacy but greater potency, than that of THC at CB1 and similar potency and efficacy at CB2. EG-018 inhibited forskolin-stimulated cAMP with similar efficacy but lower potency, compared to THC, which was likely due to high receptor density facilitating saturation of this signaling pathway. In mice, EG-018 (100 mg/kg, 30 min) administered intraperitoneally (i.p.) did not produce effects in the tetrad or drug discrimination nor did it shift THC's ED50 value in drug discrimination when administered before THC, suggesting EG-018 has negligible occupancy of brain CB1 receptors following i.p. administration. Following intravenous (i.v.) administration, EG-018 (56 mg/kg) produced hypomotility, catalepsy, and hypothermia, but only catalepsy was blocked by the selective CB1 antagonist rimonabant (3 mg/kg, i.v.). Additional studies of EG-018 and its structural analogues could provide further insight into how cannabinoids exert efficacy through the cannabinoid receptors.
Keywords: Behavior; Binding; CB(1); CB(2); Cannabinoid; Novel psychoactive substance; Signaling.
Copyright © 2020 Elsevier Inc. All rights reserved.
Figures




Similar articles
-
In vitro and in vivo pharmacology of nine novel synthetic cannabinoid receptor agonists.Pharmacol Biochem Behav. 2022 Oct;220:173467. doi: 10.1016/j.pbb.2022.173467. Epub 2022 Sep 22. Pharmacol Biochem Behav. 2022. PMID: 36154844 Free PMC article.
-
Exploring determinants of agonist efficacy at the CB1 cannabinoid receptor: Analogues of the synthetic cannabinoid receptor agonist EG-018.Pharmacol Res Perspect. 2022 Feb;10(1):e00901. doi: 10.1002/prp2.901. Pharmacol Res Perspect. 2022. PMID: 35041297 Free PMC article.
-
Differential activation of G protein-mediated signaling by synthetic cannabinoid receptor agonists.Pharmacol Res Perspect. 2020 Apr;8(2):e00566. doi: 10.1002/prp2.566. Pharmacol Res Perspect. 2020. PMID: 32101383 Free PMC article.
-
Distinct pharmacology and metabolism of K2 synthetic cannabinoids compared to Δ(9)-THC: mechanism underlying greater toxicity?Life Sci. 2014 Feb 27;97(1):45-54. doi: 10.1016/j.lfs.2013.09.017. Epub 2013 Sep 29. Life Sci. 2014. PMID: 24084047 Free PMC article. Review.
-
The Chemistry and Pharmacology of Synthetic Cannabinoid Receptor Agonists as New Psychoactive Substances: Origins.Handb Exp Pharmacol. 2018;252:165-190. doi: 10.1007/164_2018_143. Handb Exp Pharmacol. 2018. PMID: 29980914 Review.
Cited by
-
The synthetic cannabinoid 5-fluoro ABICA upregulates angiogenic markers and stimulates tube formation in human brain microvascular endothelial cells.J Taibah Univ Med Sci. 2024 Feb 1;19(2):359-371. doi: 10.1016/j.jtumed.2024.01.002. eCollection 2024 Apr. J Taibah Univ Med Sci. 2024. PMID: 38357583 Free PMC article.
-
Evaluating signaling bias for synthetic cannabinoid receptor agonists at the cannabinoid CB2 receptor.Pharmacol Res Perspect. 2023 Dec;11(6):e01157. doi: 10.1002/prp2.1157. Pharmacol Res Perspect. 2023. PMID: 38018694 Free PMC article.
-
Structure-activity relationships of valine, tert-leucine, and phenylalanine amino acid-derived synthetic cannabinoid receptor agonists related to ADB-BUTINACA, APP-BUTINACA, and ADB-P7AICA.RSC Med Chem. 2021 Oct 25;13(2):156-174. doi: 10.1039/d1md00242b. eCollection 2022 Feb 23. RSC Med Chem. 2021. PMID: 35308023 Free PMC article.
-
Synthesis and pharmacological evaluation of newly detected synthetic cannabinoid receptor agonists AB-4CN-BUTICA, MMB-4CN-BUTINACA, MDMB-4F-BUTICA, MDMB-4F-BUTINACA and their analogs.Front Psychiatry. 2022 Sep 28;13:1010501. doi: 10.3389/fpsyt.2022.1010501. eCollection 2022. Front Psychiatry. 2022. PMID: 36245876 Free PMC article.
-
Evaluation of cannabimimetic effects of selected minor cannabinoids and Terpenoids in mice.Prog Neuropsychopharmacol Biol Psychiatry. 2024 Jun 8;132:110984. doi: 10.1016/j.pnpbp.2024.110984. Epub 2024 Feb 27. Prog Neuropsychopharmacol Biol Psychiatry. 2024. PMID: 38417478 Free PMC article.
References
-
- Angerer V, Moosmann B, Franz F, Auwärter V, 2015. 5-F-cumyl-pinaca in “e-liquids” for electronic cigarettes—A new type of synthetic cannabinoid in a trendy product, 53rd Annual Meeting of the International Association of Forensic Toxicologists Meeting, Firenze, Italy.
-
- Arnott JA, Planey SL, 2012. The influence of lipophilicity in drug discovery and design. Expert Opin Drug Discov 7(10), 863–875. - PubMed
-
- Balster RL, Prescott WR, 1992. Delta 9-tetrahydrocannabinol discrimination in rats as a model for cannabis intoxication. Neurosci Biobehav Rev 16(1), 55–62. - PubMed
-
- Banister SD, Moir M, Stuart J, Kevin RC, Wood KE, Longworth M, Wilkinson SM, Beinat C, Buchanan AS, Glass M, Connor M, McGregor IS, Kassiou M, 2015. Pharmacology of Indole and Indazole Synthetic Cannabinoid Designer Drugs AB-FUBINACA, ADB-FUBINACA, AB-PINACA, ADB-PINACA, 5F-AB-PINACA, 5F-ADB-PINACA, ADBICA, and 5F-ADBICA. ACS Chem Neurosci 6(9), 1546–1559. - PubMed
-
- Barrett RL, Wiley JL, Balster RL, Martin BR, 1995. Pharmacological specificity of Δ 9-tetrahydrocannabinol discrimination in rats. Psychopharmacology 118(4), 419–424. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

