Fast field-cycling magnetic resonance detection of intracellular ultra-small iron oxide particles in vitro: Proof-of-concept
- PMID: 32248086
- PMCID: PMC7167511
- DOI: 10.1016/j.jmr.2020.106722
Fast field-cycling magnetic resonance detection of intracellular ultra-small iron oxide particles in vitro: Proof-of-concept
Abstract
Purpose: Inflammation is central in disease pathophysiology and accurate methods for its detection and quantification are increasingly required to guide diagnosis and therapy. Here we explored the ability of Fast Field-Cycling Magnetic Resonance (FFC-MR) in quantifying the signal of ultra-small superparamagnetic iron oxide particles (USPIO) phagocytosed by J774 macrophage-like cells as a proof-of-principle.
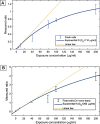
Methods: Relaxation rates were measured in suspensions of J774 macrophage-like cells loaded with USPIO (0-200 μg/ml Fe as ferumoxytol), using a 0.25 T FFC benchtop relaxometer and a human whole-body, in-house built 0.2 T FFC-MR prototype system with a custom test tube coil. Identical non-imaging, saturation recovery pulse sequence with 90° flip angle and 20 different evolution fields selected logarithmically between 80 μT and 0.2 T (3.4 kHz and 8.51 MHz proton Larmor frequency [PLF] respectively). Results were compared with imaging flow cytometry quantification of side scatter intensity and USPIO-occupied cell area. A reference colorimetric iron assay was used.
Results: The T1 dispersion curves derived from FFC-MR were excellent in detecting USPIO at all concentrations examined (0-200 μg/ml Fe as ferumoxytol) vs. control cells, p ≤ 0.001. FFC-NMR was capable of reliably detecting cellular iron content as low as 1.12 ng/µg cell protein, validated using a colorimetric assay. FFC-MR was comparable to imaging flow cytometry quantification of side scatter intensity but superior to USPIO-occupied cell area, the latter being only sensitive at exposures ≥ 10 µg/ml USPIO.
Conclusions: We demonstrated for the first time that FFC-MR is capable of quantitative assessment of intra-cellular iron which will have important implications for the use of USPIO in a variety of biological applications, including the study of inflammation.
Keywords: Fast field-cycling magnetic resonance; Inflammation; Ultrasmall superparamagnetic iron oxide particles (USPIO).
Copyright © 2020 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest DJL, DKD, HMW, RY, LC, PJR, LB, AE, DL and HA have no conflicts of interest to declare.
Figures





References
-
- Satomi T., Ogawa M., Mori I., Ishino S., Kubo K., Magata Y. Comparison of contrast agents for atherosclerosis imaging using cultured macrophages: FDG versus ultrasmall superparamagnetic iron oxide. J. Nucl. Med. 2013;54(6):999–1004. - PubMed
-
- Wang Y., Xu C., Chang Y., Zhao L., Zhang K., Zhao Y. Ultrasmall Superparamagnetic Iron Oxide Nanoparticle for T2-Weighted Magnetic Resonance Imaging. ACS Appl. Mater. Interfaces. 2017;9(34):28959–28966. - PubMed
-
- Bjornerud A., Johansson L. The utility of superparamagnetic contrast agents in MRI: theoretical consideration and applications in the cardiovascular system. NMR Biomed. 2004;17(7):465–477. - PubMed
-
- Rogers W.J., Basu P. Factors regulating macrophage endocytosis of nanoparticles: implications for targeted magnetic resonance plaque imaging. Atherosclerosis. 2005;178(1):67–73. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

