GAD-alum immunotherapy in type 1 diabetes expands bifunctional Th1/Th2 autoreactive CD4 T cells
- PMID: 32248243
- PMCID: PMC7228993
- DOI: 10.1007/s00125-020-05130-7
GAD-alum immunotherapy in type 1 diabetes expands bifunctional Th1/Th2 autoreactive CD4 T cells
Abstract
Aims/hypothesis: Antigen-specific therapy aims to modify inflammatory T cell responses in type 1 diabetes and restore immune tolerance. One strategy employs GAD65 conjugated to aluminium hydroxide (GAD-alum) to take advantage of the T helper (Th)2-biasing adjuvant properties of alum and thereby regulate pathological Th1 autoimmunity. We explored the cellular and molecular mechanism of GAD-alum action in the setting of a previously reported randomised placebo-controlled clinical trial conducted by Type 1 Diabetes TrialNet.
Methods: In the clinical trial conducted by Type 1 Diabetes TrialNet, participants were immunised with 20 μg GAD-alum (twice or three times) or alum alone and peripheral blood mononuclear cell samples were banked at baseline and post treatment. In the present study, GAD-specific T cell responses were measured in these samples and GAD-specific T cell lines and clones were generated, which were then further characterised.
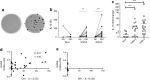
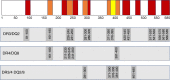
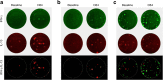
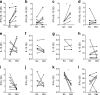
Results: At day 91 post immunisation, we detected GAD-specific IL-13+ CD4 T cell responses significantly more frequently in participants immunised with GAD-alum (71% and 94% treated twice or three times, respectively) compared with those immunised with alum alone (38%; p = 0.003 and p = 0.0002, respectively) accompanied by high secreted levels of IL-13, IL-4 and IL-5, confirming a GAD-specific, GAD-alum-induced Th2 response. Of note, GAD-specific, IL-13+ CD4 T cells observed after immunisation co-secreted IFN-γ, displaying a bifunctional Th1/Th2 phenotype. Single-cell transcriptome analysis identified IL13 and IFNG expression in concert with the canonical Th2 and Th1 transcription factor genes GATA3 and TBX21, respectively. T cell receptor β-chain (TCRB) CDR3 regions of GAD-specific bifunctional T cells were identified in circulating naive and central memory CD4 T cell pools of non-immunised participants with new-onset type 1 diabetes and healthy individuals, suggesting the potential for bifunctional responses to be generated de novo by GAD-alum immunisation or via expansion from an existing public repertoire.
Conclusions/interpretation: GAD-alum immunisation activates and propagates GAD-specific CD4 T cells with a distinctive bifunctional phenotype, the functional analysis of which might be important in understanding therapeutic responses.
Keywords: Autoreactive; Epitopes; GAD; GAD-alum; Glutamic acid decarboxylase; IL-13; Immunotherapy; T cell receptor; T cells; TCR; Th2; TrialNet.
Figures


References
-
- Elding Larsson H, Lundgren M, Jonsdottir B, Cuthbertson D, Krischer J, DiAPREV-IT Study Group Safety and efficacy of autoantigen-specific therapy with 2 doses of alum-formulated glutamate decarboxylase in children with multiple islet autoantibodies and risk for type 1 diabetes: a randomized clinical trial. Pediatr Diabetes. 2018;19(3):410–419. doi: 10.1111/pedi.12611. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- U01 DK103153/DK/NIDDK NIH HHS/United States
- U01 DK061010/DK/NIDDK NIH HHS/United States
- U01 DK060916/DK/NIDDK NIH HHS/United States
- U01 DK061034/DK/NIDDK NIH HHS/United States
- U01 DK061036/DK/NIDDK NIH HHS/United States
- U01 DK061040/DK/NIDDK NIH HHS/United States
- U01 DK061041/DK/NIDDK NIH HHS/United States
- U01 DK061042/DK/NIDDK NIH HHS/United States
- U01 DK061055/DK/NIDDK NIH HHS/United States
- U01 DK061058/DK/NIDDK NIH HHS/United States
- U01 DK084536/DK/NIDDK NIH HHS/United States
- U01 DK085453/DK/NIDDK NIH HHS/United States
- U01 DK085461/DK/NIDDK NIH HHS/United States
- U01 DK085463/DK/NIDDK NIH HHS/United States
- U01 DK085466/DK/NIDDK NIH HHS/United States
- U01 DK085499/DK/NIDDK NIH HHS/United States
- U01 DK085505/DK/NIDDK NIH HHS/United States
- U01 DK085509/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Research Materials

