The Impact of Elastic Deformations of the Extracellular Matrix on Cell Migration
- PMID: 32248312
- PMCID: PMC7128007
- DOI: 10.1007/s11538-020-00721-2
The Impact of Elastic Deformations of the Extracellular Matrix on Cell Migration
Abstract
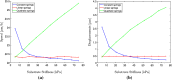
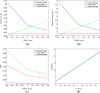
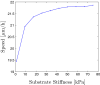
The mechanical properties of the extracellular matrix, in particular its stiffness, are known to impact cell migration. In this paper, we develop a mathematical model of a single cell migrating on an elastic matrix, which accounts for the deformation of the matrix induced by forces exerted by the cell, and investigate how the stiffness impacts the direction and speed of migration. We model a cell in 1D as a nucleus connected to a number of adhesion sites through elastic springs. The cell migrates by randomly updating the position of its adhesion sites. We start by investigating the case where the cell springs are constant, and then go on to assuming that they depend on the matrix stiffness, on matrices of both uniform stiffness as well as those with a stiffness gradient. We find that the assumption that cell springs depend on the substrate stiffness is necessary and sufficient for an efficient durotactic response. We compare simulations to recent experimental observations of human cancer cells exhibiting durotaxis, which show good qualitative agreement.
Keywords: Cell migration; Durotaxis; Mathematical modeling; Stochastic simulation.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures



Similar articles
-
Mathematical modelling of cell migration: stiffness dependent jump rates result in durotaxis.J Math Biol. 2019 Jun;78(7):2289-2315. doi: 10.1007/s00285-019-01344-5. Epub 2019 Apr 10. J Math Biol. 2019. PMID: 30972438 Free PMC article.
-
Competing elastic and viscous gradients determine directional cell migration.Math Biosci. 2025 Feb;380:109362. doi: 10.1016/j.mbs.2024.109362. Epub 2024 Dec 17. Math Biosci. 2025. PMID: 39701208
-
A Cellular Potts Model of single cell migration in presence of durotaxis.Math Biosci. 2016 May;275:57-70. doi: 10.1016/j.mbs.2016.02.011. Epub 2016 Mar 9. Math Biosci. 2016. PMID: 26968932
-
Probing mechanical principles of focal contacts in cell-matrix adhesion with a coupled stochastic-elastic modelling framework.J R Soc Interface. 2011 Sep 7;8(62):1217-32. doi: 10.1098/rsif.2011.0157. Epub 2011 Jun 1. J R Soc Interface. 2011. PMID: 21632610 Free PMC article. Review.
-
Durotaxis.Curr Biol. 2020 May 4;30(9):R383-R387. doi: 10.1016/j.cub.2020.03.051. Curr Biol. 2020. PMID: 32369745 Review.
Cited by
-
Biophysical Control of the Glioblastoma Immunosuppressive Microenvironment: Opportunities for Immunotherapy.Bioengineering (Basel). 2024 Jan 18;11(1):93. doi: 10.3390/bioengineering11010093. Bioengineering (Basel). 2024. PMID: 38247970 Free PMC article. Review.
-
Computational models of migration modes improve our understanding of metastasis.APL Bioeng. 2020 Nov 5;4(4):041505. doi: 10.1063/5.0023748. eCollection 2020 Dec. APL Bioeng. 2020. PMID: 33195959 Free PMC article. Review.
-
Mechanics of Pollen Tube Elongation: A Perspective.Front Plant Sci. 2020 Oct 20;11:589712. doi: 10.3389/fpls.2020.589712. eCollection 2020. Front Plant Sci. 2020. PMID: 33193543 Free PMC article. Review.
-
Penetrating the ultra-tough yeast cell wall with finite element analysis model-aided design of microtools.iScience. 2024 Mar 16;27(4):109503. doi: 10.1016/j.isci.2024.109503. eCollection 2024 Apr 19. iScience. 2024. PMID: 38591007 Free PMC article.
-
An Endothelial Cell Is Not Simply an Endothelial Cell.Stem Cells Dev. 2024 Oct;33(19-20):517-527. doi: 10.1089/scd.2024.0088. Epub 2024 Aug 9. Stem Cells Dev. 2024. PMID: 39030822 Review.
References
-
- Alberts B. Molecular biology of the cell. New York: Garland Science; 2017.
-
- Allena Rachele, Scianna Marco, Preziosi Luigi. A cellular potts model of single cell migration in presence of durotaxis. Math Biosci. 2016;275:57–70. - PubMed
-
- Carter Stephen B. Haptotaxis and the mechanism of cell motility. Nature. 1967;213(5073):256. - PubMed
-
- Dallon JC, Evans Emily J, Grant Christopher P, Smith William V. Cell speed is independent of force in a mathematical model of amoeboidal cell motion with random switching terms. Math Biosci. 2013;246(1):1–7. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

