DNA-Scaffolded Proximity Assembly and Confinement of Multienzyme Reactions
- PMID: 32248317
- PMCID: PMC7127875
- DOI: 10.1007/s41061-020-0299-3
DNA-Scaffolded Proximity Assembly and Confinement of Multienzyme Reactions
Abstract
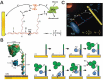
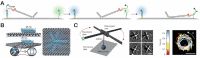
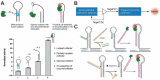
Cellular functions rely on a series of organized and regulated multienzyme cascade reactions. The catalytic efficiencies of these cascades depend on the precise spatial organization of the constituent enzymes, which is optimized to facilitate substrate transport and regulate activities. Mimicry of this organization in a non-living, artificial system would be very useful in a broad range of applications-with impacts on both the scientific community and society at large. Self-assembled DNA nanostructures are promising applications to organize biomolecular components into prescribed, multidimensional patterns. In this review, we focus on recent progress in the field of DNA-scaffolded assembly and confinement of multienzyme reactions. DNA self-assembly is exploited to build spatially organized multienzyme cascades with control over their relative distance, substrate diffusion paths, compartmentalization and activity actuation. The combination of addressable DNA assembly and multienzyme cascades can deliver breakthroughs toward the engineering of novel synthetic and biomimetic reactors.
Keywords: Biomimetic systems; DNA nanotechnology; DNA scaffolded assembly; Enzyme encapsulation; Enzyme immobilization; Enzyme regulation; Multienzyme cascade; Synthetic reactors.
Conflict of interest statement
The authors declare no competing financial interest.
Figures











Similar articles
-
Biomimetic Compartments Scaffolded by Nucleic Acid Nanostructures.Small. 2019 Jun;15(26):e1900256. doi: 10.1002/smll.201900256. Epub 2019 Mar 18. Small. 2019. PMID: 30884139 Review.
-
DNA Nanoscaffolds for Multienzyme Systems Assembly.Methods Mol Biol. 2022;2487:93-112. doi: 10.1007/978-1-0716-2269-8_6. Methods Mol Biol. 2022. PMID: 35687231
-
Assembly of multienzyme complexes on DNA nanostructures.Nat Protoc. 2016 Nov;11(11):2243-2273. doi: 10.1038/nprot.2016.139. Epub 2016 Oct 20. Nat Protoc. 2016. PMID: 27763626
-
Spatially-interactive biomolecular networks organized by nucleic acid nanostructures.Acc Chem Res. 2012 Aug 21;45(8):1215-26. doi: 10.1021/ar200295q. Epub 2012 May 29. Acc Chem Res. 2012. PMID: 22642503 Free PMC article. Review.
-
Nano- and Microscale Confinements in DNA-Scaffolded Enzyme Cascade Reactions.Small. 2024 Jan;20(4):e2304578. doi: 10.1002/smll.202304578. Epub 2023 Sep 21. Small. 2024. PMID: 37732702
Cited by
-
Precise surface functionalization of PLGA particles for human T cell modulation.Nat Protoc. 2023 Nov;18(11):3289-3321. doi: 10.1038/s41596-023-00887-8. Epub 2023 Oct 18. Nat Protoc. 2023. PMID: 37853157 Free PMC article.
-
Mechanistic Aspects for the Modulation of Enzyme Reactions on the DNA Scaffold.Molecules. 2022 Sep 24;27(19):6309. doi: 10.3390/molecules27196309. Molecules. 2022. PMID: 36234845 Free PMC article. Review.
-
A Robust and Efficient Method to Purify DNA-Scaffolded Nanostructures by Gravity-Driven Size Exclusion Chromatography.Langmuir. 2024 Apr 23;40(16):8365-8372. doi: 10.1021/acs.langmuir.3c03778. Epub 2024 Apr 10. Langmuir. 2024. PMID: 38600821 Free PMC article.
-
Digital nanoreactors to control absolute stoichiometry and spatiotemporal behavior of DNA receptors within lipid bilayers.Nat Commun. 2023 Mar 20;14(1):1532. doi: 10.1038/s41467-023-36996-x. Nat Commun. 2023. PMID: 36941256 Free PMC article.
-
Nano-sandwich composite by kinetic trapping assembly from protein and nucleic acid.Nucleic Acids Res. 2021 Sep 27;49(17):10098-10105. doi: 10.1093/nar/gkab797. Nucleic Acids Res. 2021. PMID: 34500473 Free PMC article.
References
-
- Schomburg I, Chang A, Placzek S, Söhngen C, Rother M, Lang M, Munaretto C, Ulas S, Stelzer M, Grote A, Scheer M, Schomburg D. BRENDA in 2013: integrated reactions, kinetic data, enzyme function data, improved disease classification: new options and contents in BRENDA. Nucleic Acids Res. 2013;41:D764–D772. - PMC - PubMed
-
- Roach PJ. Functional significance of enzyme cascade systems. Trends Biochem Sci. 1977;2:87–90.
-
- Savage DF, Afonso B, Chen AH, Silver PA. Spatially ordered dynamics of the bacterial carbon fixation machinery. Science. 2010;327:1258–1261. - PubMed
-
- Srere PA, Mosbach K. Metabolic compartmentation: symbiotic, organellar, multienzymic, and microenvironmental. Annu Rev Microbiol. 1974;28:61–84. - PubMed
-
- Bayer EA, Belaich JP, Shoham Y, Lamed R. The cellulosomes: multienzyme machines for degradation of plant cell wall polysaccharides. Annu Rev Microbiol. 2004;58:521–554. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
