Absorption, distribution, metabolism and excretion of molidustat in healthy participants
- PMID: 32248614
- PMCID: PMC7496954
- DOI: 10.1111/bcpt.13409
Absorption, distribution, metabolism and excretion of molidustat in healthy participants
Abstract
The absorption, distribution, metabolism and excretion of molidustat were investigated in healthy male participants. In study 1, a mass balance study, radiolabelled molidustat 25 mg (3.57 MBq) was administered as an oral solution (n = 4). Following rapid absorption, molidustat-related radioactivity was predominantly distributed in plasma rather than in red blood cells. The total recovery of the administered radioactivity was 97.0%, which was mainly excreted renally (90.7%). Metabolite M-1, produced by N-glucuronidation, was the dominant component in plasma (80.2% of the area under the concentration-time curve for total radioactivity) and was primarily excreted via urine (~85% of dose). Only minor amounts of unchanged molidustat were excreted in urine (~4%) and faeces (~6%). Study 2 investigated the absolute bioavailability and pharmacodynamics of molidustat (part 1, n = 12; part 2, n = 16). Orally administered molidustat immediate release tablets had an absolute bioavailability of 59%. Following intravenous administration (1, 5 and 25 mg), total body clearance of molidustat was 28.7-34.5 L/h and volume of distribution at steady state was 39.3-50.0 L. All doses of molidustat transiently elevated endogenous erythropoietin levels, irrespective of the route of administration. Molidustat was considered safe and well tolerated at the administered doses.
Keywords: ADME (absorption; biotransformation; disposition; erythropoietin; excretion); metabolism.
© 2020 The Authors. Basic & Clinical Pharmacology & Toxicology published by John Wiley & Sons Ltd on behalf of Nordic Association for the Publication of BCPT (former Nordic Pharmacological Society).
Conflict of interest statement
Silvia Lentini, Dorina van der Mey, Armin Kern, Uwe Thuss, Andreas Kaiser and Michael Gerisch are employed by Bayer AG. Kumi Matsuno is employed by Bayer Yakuhin Ltd. Dorina van der Mey and Michael Gerisch have stock options for Bayer AG, which are unrelated to primary employment. Medical writing support was provided by Cerys Evans, PhD, of Oxford PharmaGenesis, Oxford, UK, and was funded by Bayer Yakuhin Ltd.
Figures


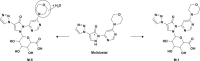

Similar articles
-
Drug-drug interaction of atazanavir on UGT1A1-mediated glucuronidation of molidustat in human.Basic Clin Pharmacol Toxicol. 2021 Mar;128(3):511-524. doi: 10.1111/bcpt.13538. Epub 2020 Dec 12. Basic Clin Pharmacol Toxicol. 2021. PMID: 33232579 Free PMC article.
-
Assessment of the absorption, metabolism and absolute bioavailability of maraviroc in healthy male subjects.Br J Clin Pharmacol. 2008 Apr;65 Suppl 1(Suppl 1):60-7. doi: 10.1111/j.1365-2125.2008.03137.x. Br J Clin Pharmacol. 2008. PMID: 18333867 Free PMC article. Clinical Trial.
-
First-in-man-proof of concept study with molidustat: a novel selective oral HIF-prolyl hydroxylase inhibitor for the treatment of renal anaemia.Br J Clin Pharmacol. 2018 Jul;84(7):1557-1565. doi: 10.1111/bcp.13584. Epub 2018 May 14. Br J Clin Pharmacol. 2018. PMID: 29575006 Free PMC article. Clinical Trial.
-
Absorption, distribution, metabolism and excretion of glucosamine sulfate. A review.Arzneimittelforschung. 2001 Sep;51(9):699-725. doi: 10.1055/s-0031-1300105. Arzneimittelforschung. 2001. PMID: 11642003 Review.
-
Cefetamet pivoxil clinical pharmacokinetics.Clin Pharmacokinet. 1993 Sep;25(3):172-88. doi: 10.2165/00003088-199325030-00002. Clin Pharmacokinet. 1993. PMID: 8222459 Review.
Cited by
-
Bioanalysis, Analysis, Chemistry, and Pharmacological Aspects of Hypoxia-Inducible Factor Prolyl Hydroxylase Inhibitors.Curr Top Med Chem. 2025;25(12):1451-1466. doi: 10.2174/0115680266324419241227102847. Curr Top Med Chem. 2025. PMID: 39791153 Review.
-
Simultaneous detection of three hypoxia-inducible factor stabilizers-molidustat, roxadustat, and vadadustat-in multiple keratinized matrices and its application in a doping context.Drug Test Anal. 2025 May;17(5):647-654. doi: 10.1002/dta.3771. Epub 2024 Jul 11. Drug Test Anal. 2025. PMID: 38992954 Free PMC article.
-
HIF-α Prolyl Hydroxylase Inhibitors and Their Implications for Biomedicine: A Comprehensive Review.Biomedicines. 2021 Apr 24;9(5):468. doi: 10.3390/biomedicines9050468. Biomedicines. 2021. PMID: 33923349 Free PMC article. Review.
-
Metabolite Profiling of Meridianin C In Vivo of Rat by UHPLC/Q-TOF MS.J Anal Methods Chem. 2021 Oct 21;2021:1382421. doi: 10.1155/2021/1382421. eCollection 2021. J Anal Methods Chem. 2021. PMID: 34721922 Free PMC article.
-
Drug-drug interaction of atazanavir on UGT1A1-mediated glucuronidation of molidustat in human.Basic Clin Pharmacol Toxicol. 2021 Mar;128(3):511-524. doi: 10.1111/bcpt.13538. Epub 2020 Dec 12. Basic Clin Pharmacol Toxicol. 2021. PMID: 33232579 Free PMC article.
References
-
- Astor BC, Muntner P, Levin A, Eustace JA, Coresh J. Association of kidney function with anemia: the Third National Health and Nutrition Examination Survey (1988–1994). Arch Intern Med. 2002;162:1401‐1408. - PubMed
-
- Hsu CY, McCulloch CE, Curhan GC. Epidemiology of anemia associated with chronic renal insufficiency among adults in the United States: results from the Third National Health and Nutrition Examination Survey. J Am Soc Nephrol. 2002;13:504‐510. - PubMed
-
- Yamamoto H, Nishi S, Tomo T, et al. 2015 Japanese Society for Dialysis Therapy: guidelines for renal anemia in chronic kidney disease. Renal Replacement Therapy 2017;3:36.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

