Absorption, distribution, metabolism and excretion of molidustat in healthy participants
- PMID: 32248614
- PMCID: PMC7496954
- DOI: 10.1111/bcpt.13409
Absorption, distribution, metabolism and excretion of molidustat in healthy participants
Abstract
The absorption, distribution, metabolism and excretion of molidustat were investigated in healthy male participants. In study 1, a mass balance study, radiolabelled molidustat 25 mg (3.57 MBq) was administered as an oral solution (n = 4). Following rapid absorption, molidustat-related radioactivity was predominantly distributed in plasma rather than in red blood cells. The total recovery of the administered radioactivity was 97.0%, which was mainly excreted renally (90.7%). Metabolite M-1, produced by N-glucuronidation, was the dominant component in plasma (80.2% of the area under the concentration-time curve for total radioactivity) and was primarily excreted via urine (~85% of dose). Only minor amounts of unchanged molidustat were excreted in urine (~4%) and faeces (~6%). Study 2 investigated the absolute bioavailability and pharmacodynamics of molidustat (part 1, n = 12; part 2, n = 16). Orally administered molidustat immediate release tablets had an absolute bioavailability of 59%. Following intravenous administration (1, 5 and 25 mg), total body clearance of molidustat was 28.7-34.5 L/h and volume of distribution at steady state was 39.3-50.0 L. All doses of molidustat transiently elevated endogenous erythropoietin levels, irrespective of the route of administration. Molidustat was considered safe and well tolerated at the administered doses.
Keywords: ADME (absorption; biotransformation; disposition; erythropoietin; excretion); metabolism.
© 2020 The Authors. Basic & Clinical Pharmacology & Toxicology published by John Wiley & Sons Ltd on behalf of Nordic Association for the Publication of BCPT (former Nordic Pharmacological Society).
Conflict of interest statement
Silvia Lentini, Dorina van der Mey, Armin Kern, Uwe Thuss, Andreas Kaiser and Michael Gerisch are employed by Bayer AG. Kumi Matsuno is employed by Bayer Yakuhin Ltd. Dorina van der Mey and Michael Gerisch have stock options for Bayer AG, which are unrelated to primary employment. Medical writing support was provided by Cerys Evans, PhD, of Oxford PharmaGenesis, Oxford, UK, and was funded by Bayer Yakuhin Ltd.
Figures


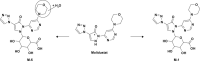

References
-
- Astor BC, Muntner P, Levin A, Eustace JA, Coresh J. Association of kidney function with anemia: the Third National Health and Nutrition Examination Survey (1988–1994). Arch Intern Med. 2002;162:1401‐1408. - PubMed
-
- Hsu CY, McCulloch CE, Curhan GC. Epidemiology of anemia associated with chronic renal insufficiency among adults in the United States: results from the Third National Health and Nutrition Examination Survey. J Am Soc Nephrol. 2002;13:504‐510. - PubMed
-
- Yamamoto H, Nishi S, Tomo T, et al. 2015 Japanese Society for Dialysis Therapy: guidelines for renal anemia in chronic kidney disease. Renal Replacement Therapy 2017;3:36.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

