Thrombolysis With Alteplase at 0.6 mg/kg for Stroke With Unknown Time of Onset: A Randomized Controlled Trial
- PMID: 32248771
- PMCID: PMC7185058
- DOI: 10.1161/STROKEAHA.119.028127
Thrombolysis With Alteplase at 0.6 mg/kg for Stroke With Unknown Time of Onset: A Randomized Controlled Trial
Abstract
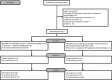
Background and Purpose- We assessed whether lower-dose alteplase at 0.6 mg/kg is efficacious and safe for acute fluid-attenuated inversion recovery-negative stroke with unknown time of onset. Methods- This was an investigator-initiated, multicenter, randomized, open-label, blinded-end point trial. Patients met the standard indication criteria for intravenous thrombolysis other than a time last-known-well >4.5 hours (eg, wake-up stroke). Patients were randomly assigned (1:1) to receive alteplase at 0.6 mg/kg or standard medical treatment if magnetic resonance imaging showed acute ischemic lesion on diffusion-weighted imaging and no marked corresponding hyperintensity on fluid-attenuated inversion recovery. The primary outcome was a favorable outcome (90-day modified Rankin Scale score of 0-1). Results- Following the early stop and positive results of the WAKE-UP trial (Efficacy and Safety of MRI-Based Thrombolysis in Wake-Up Stroke), this trial was prematurely terminated with 131 of the anticipated 300 patients (55 women; mean age, 74.4±12.2 years). Favorable outcome was comparable between the alteplase group (32/68, 47.1%) and the control group (28/58, 48.3%; relative risk [RR], 0.97 [95% CI, 0.68-1.41]; P=0.892). Symptomatic intracranial hemorrhage within 22 to 36 hours occurred in 1/71 and 0/60 (RR, infinity [95% CI, 0.06 to infinity]; P>0.999), respectively. Death at 90 days occurred in 2/71 and 2/60 (RR, 0.85 [95% CI, 0.06-12.58]; P>0.999), respectively. Conclusions- No difference in favorable outcome was seen between alteplase and control groups among patients with ischemic stroke with unknown time of onset. The safety of alteplase at 0.6 mg/kg was comparable to that of standard treatment. Early study termination precludes any definitive conclusions. Registration- URL: https://www.clinicaltrials.gov; Unique identifier: NCT02002325.
Keywords: control groups; informed consent; intracranial hemorrhages; magnetic resonance imaging; stroke, acute; tissue-type plasminogen activator.
Figures


References
-
- Thomalla G, Simonsen CZ, Boutitie F, Andersen G, Berthezene Y, Cheng B, et al. WAKE-UP Investigators. MRI-Guided thrombolysis for stroke with unknown time of onset. N Engl J Med. 2018;379:611–622. doi: 10.1056/NEJMoa1804355. - PubMed
-
- Yamaguchi T, Mori E, Minematsu K, Nakagawara J, Hashi K, Saito I, et al. Japan Alteplase Clinical Trial (J-ACT) Group. Alteplase at 0.6 mg/kg for acute ischemic stroke within 3 hours of onset: Japan Alteplase Clinical Trial (J-ACT). Stroke. 2006;37:1810–1815. doi: 10.1161/01.STR.0000227191.01792.e3. - PubMed
-
- Nezu T, Koga M, Kimura K, Shiokawa Y, Nakagawara J, Furui E, et al. Pretreatment ASPECTS on DWI predicts 3-month outcome following rt-PA: SAMURAI rt-PA Registry. Neurology. 2010;75:555–561. doi: 10.1212/WNL.0b013e3181eccf78. - PubMed
-
- Toyoda K, Koga M, Naganuma M, Shiokawa Y, Nakagawara J, Furui E, et al. Stroke Acute Management With Urgent Risk-Factor Assessment and Improvement Study Investigators. Routine use of intravenous low-dose recombinant tissue plasminogen activator in Japanese patients: general outcomes and prognostic factors from the SAMURAI register. Stroke. 2009;40:3591–3595. doi: 10.1161/STROKEAHA.109.562991. - PubMed
-
- Minematsu K, Toyoda K, Hirano T, Kimura K, Kondo R, Mori E, et al. Japan Stroke Society. Guidelines for the intravenous application of recombinant tissue-type plasminogen activator (alteplase), the second edition, October 2012: a guideline from the Japan Stroke Society. J Stroke Cerebrovasc Dis. 2013;22:571–600. doi: 10.1016/j.jstrokecerebrovasdis.2013.04.001. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

