The impact of hyperpolarization-activated cyclic nucleotide-gated (HCN) and voltage-gated potassium KCNQ/Kv7 channels on primary microglia function
- PMID: 32248813
- PMCID: PMC7132998
- DOI: 10.1186/s12974-020-01779-4
The impact of hyperpolarization-activated cyclic nucleotide-gated (HCN) and voltage-gated potassium KCNQ/Kv7 channels on primary microglia function
Abstract
Background: Microglia are essential to maintain cell homeostasis in the healthy brain and are activated after brain injury. Upon activation, microglia polarize towards different phenotypes. The course of microglia activation is complex and depends on signals in the surrounding milieu. Recently, it has been suggested that microglia respond to ion currents, as a way of regulating their activity and function.
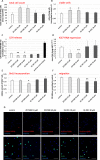
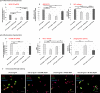
Methods and results: Under the hypothesis that HCN and KCNQ/Kv7 channels impact on microglia, we studied primary rat microglia in the presence or absence of specific pharmacological blockade or RNA silencing. Primary microglia expressed the subunits HCN1-4, Kv7.2, Kv7.3, and Kv7.5. The expression of HCN2, as well as Kv7.2 and Kv7.3, varied among different microglia phenotypes. The pharmacological blockade of HCN channels by ZD7288 resulted in cell depolarization with slowly rising intracellular calcium levels, leading to enhanced survival and reduced proliferation rates of resting microglia. Furthermore, ZD7288 treatment, as well as knockdown of HCN2 RNA by small interfering RNA, resulted in an attenuation of later microglia activation-both towards the anti- and pro-inflammatory phenotype. However, HCN channel inhibition enhanced the phagocytic capacity of IL4-stimulated microglia. Blockade of Kv7/KCNQ channel by XE-991 exclusively inhibited the migratory capacity of resting microglia.
Conclusion: These observations suggest that the HCN current contributes to various microglia functions and impacts on the course of microglia activation, while the Kv7/KCNQ channels affect microglia migration. Characterizing the role of HCN channels in microglial functioning may offer new therapeutic approaches for targeted modulation of neuroinflammation as a hallmark of various neurological disorders.
Keywords: Cerebral ischemia; Ih-current; Ion channel; Microglia activation; Microglia phenotype; Migration; Neuroinflammation; Phagocytosis; Voltage sensor probes; XE-991; ZD7288; siHCN2.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



References
-
- Walberer M, Jantzen SU, Backes H, Rueger MA, Keuters MH, Neumaier B, Hoehn M, Fink GR, Graf R, Schroeter M. In-vivo detection of inflammation and neurodegeneration in the chronic phase after permanent embolic stroke in rats. Brain Res. 2014;1581:80–88. doi: 10.1016/j.brainres.2014.05.030. - DOI - PubMed
-
- Walberer M, Rueger MA, Simard ML, Emig B, Jander S, Fink GR, Schroeter M. Dynamics of neuroinflammation in the macrosphere model of arterio-arterial embolic focal ischemia: an approximation to human stroke patterns. Exp Transl Stroke Med. 2010;2(1):22. doi: 10.1186/2040-7378-2-22. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

