Health and economic burden of respiratory syncytial virus (RSV) disease and the cost-effectiveness of potential interventions against RSV among children under 5 years in 72 Gavi-eligible countries
- PMID: 32248817
- PMCID: PMC7132892
- DOI: 10.1186/s12916-020-01537-6
Health and economic burden of respiratory syncytial virus (RSV) disease and the cost-effectiveness of potential interventions against RSV among children under 5 years in 72 Gavi-eligible countries
Abstract
Background: Respiratory syncytial virus (RSV) frequently causes acute lower respiratory infection in children under 5, representing a high burden in Gavi-eligible countries (mostly low-income and lower-middle-income). Since multiple RSV interventions, including vaccines and monoclonal antibody (mAb) candidates, are under development, we aim to evaluate the key drivers of the cost-effectiveness of maternal vaccination and infant mAb for 72 Gavi countries.
Methods: A static Multi-Country Model Application for RSV Cost-Effectiveness poLicy (MCMARCEL) was developed to follow RSV-related events monthly from birth until 5 years of age. MCMARCEL was parameterised using country- and age-specific demographic, epidemiological, and cost data. The interventions' level and duration of effectiveness were guided by the World Health Organization's preferred product characteristics and other literature. Maternal vaccination and mAb were assumed to require single-dose administration at prices assumed to align with other Gavi-subsidised technologies. The effectiveness and the prices of the interventions were simultaneously varied in extensive scenario analyses. Disability-adjusted life years (DALYs) were the primary health outcomes for cost-effectiveness, integrated with probabilistic sensitivity analyses and Expected Value of Partially Perfect Information analysis.
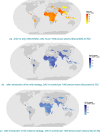
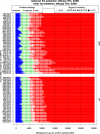
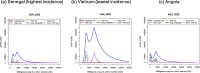
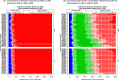
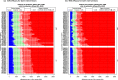
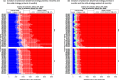
Results: The RSV-associated disease burden among children in these 72 countries is estimated at an average of 20.8 million cases, 1.8 million hospital admissions, 40 thousand deaths, 1.2 million discounted DALYs, and US$611 million discounted direct costs. Strategy 'mAb' is more effective due to its assumed longer duration of protection versus maternal vaccination, but it was also assumed to be more expensive. Given all parameterised uncertainty, the optimal strategy of choice tends to change for increasing willingness to pay (WTP) values per DALY averted from the current situation to maternal vaccination (at WTP > US$1000) to mAB (at WTP > US$3500). The age-specific proportions of cases that are hospitalised and/or die cause most of the uncertainty in the choice of optimal strategy. Results are broadly similar across countries.
Conclusions: Both the maternal and mAb strategies need to be competitively priced to be judged as relatively cost-effective. Information on the level and duration of protection is crucial, but also more and better disease burden evidence-especially on RSV-attributable hospitalisation and death rates-is needed to support policy choices when novel RSV products become available.
Keywords: Cost-effectiveness analysis; Disease burden; Expected Value of Partially Perfect Information; Low-income countries and lower-middle-income countries; Maternal vaccination; Monoclonal antibody; Probabilistic sensitivity analysis; Respiratory syncytial virus.
Conflict of interest statement
This article is the result of work conducted independently by the authors. As part of standard procedures of the Innovative Medicines Initiative (IMI) project, all academic and EFPIA members of the Respiratory Syncytial Virus Consortium in Europe (RESCEU) steering committee were given the opportunity to comment on this manuscript prior to submission. In RESCEU’s steering committee, employees of five pharmaceutical companies (Novavax, Sanofi Pasteur, GSK, Pfizer, and Johnson & Johnson) take representation for EFPIA. While the pharmaceutical companies were allowed to read and comment on the manuscript, they did not influence the final decision to publish nor did they have control over the contents of the publication.
This entire manuscript and the additional file are written independently by the authors. None of the authors have received any forms of pecuniary or other support from the pharmaceutical industry.
PB declares that a part-time (35%) university chair (occupied by Niel Hens) in the Centre for Health Economics Research and Modelling Infectious Diseases at the University of Antwerp was partially supported in 2009–2018 by a recurring gift from Pfizer, and this chair has also been partially supported by GSK in 2017.
The University of Antwerp also received compensation for PB and LW’s attendance at two meetings of a Belgian advisory board on economic evaluations of vaccines convened by Pfizer in 2019, outside of this submission work. The clinical trials centre (to which none of the authors belong) at the University of Antwerp conducts numerous vaccine trials and epidemiological studies for vaccine developers and other stakeholders in global public health.
Figures
References
-
- Nair H, Nokes DJ, Gessner BD, Dherani M, Madhi SA, Singleton RJ, O'Brien KL, Roca A, Wright PF, Bruce N, et al. Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: a systematic review and meta-analysis. Lancet. 2010;375(9725):1545–1555. doi: 10.1016/S0140-6736(10)60206-1. - DOI - PMC - PubMed
-
- Shi T, McAllister DA, O'Brien KL, Simoes EAF, Madhi SA, Gessner BD, Polack FP, Balsells E, Acacio S, Aguayo C, et al. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in young children in 2015: a systematic review and modelling study. Lancet. 2017;390(10098):946–958. doi: 10.1016/S0140-6736(17)30938-8. - DOI - PMC - PubMed
-
- American Academy of Pediatrics Committee On Infectious D, American Academy Of Pediatrics Bronchiolitis Guidelines C Updated guidance for palivizumab prophylaxis among infants and young children at increased risk of hospitalization for respiratory syncytial virus infection. Pediatrics. 2014;134(2):415–420. doi: 10.1542/peds.2014-1665. - DOI - PubMed
-
- ClinicalTrials.gov . A study to determine the safety and efficacy of the RSV F vaccine to protect infants via maternal immunization [NCT02624947] 2018.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

