The USP18 cysteine protease promotes HBV production independent of its protease activity
- PMID: 32248821
- PMCID: PMC7133002
- DOI: 10.1186/s12985-020-01304-2
The USP18 cysteine protease promotes HBV production independent of its protease activity
Abstract
Background: Hepatitis B virus (HBV) infection remains as one of the major public health problems in the world. Type I interferon (IFN) plays an essential role in antiviral defense by induced expression of a few hundred interferon stimulated genes (ISGs), including ubiquitin-specific protease 18 (USP18). The expression level of USP18 was elevated in the pretreatment liver tissues of chronic hepatitis B(CHB) patients who did not respond to IFN treatment. Thus, this study was designed to investigate the effects of USP18 on HBV replication/production.
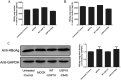
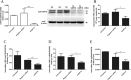
Methods: The levels of wild type USP18(WT-USP18) and USP18 catalytically inactive form C64S were up-regulated by plasmids transfection in HepAD38 cells, respectively. Real-time PCR and ELISA were used to quantify HBV replication. Type I IFN signaling pathway was monitored at three levels: p-STAT1 (western Blot), interferon stimulated response element (ISRE) activity (dual luciferase assay) and ISGs expression (real time PCR).
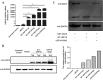
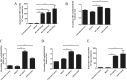
Results: Our data demonstrated that overexpression of either WT-USP18 or USP18-C64S inactive mutant increased the intracellular viral pgRNA, total DNA, cccDNA, as well as HBV DNA levels in the culture supernatant, while silencing USP18 led to opposite effect on HBV production. In addition, upregulated WT-USP18 or USP18-C64S suppressed ISRE activity and the expression levels of p-STAT1 and ISGs.
Conclusion: USP18 promoted HBV replication via inhibiting type I IFN signaling pathway, which was independent of its protease activity.
Keywords: HBV; Interferon; Persistent infection; Type I IFN signaling pathway; USP18.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

Similar articles
-
Interleukin-17A pretreatment attenuates the anti-hepatitis B virus efficacy of interferon-alpha by reducing activation of the interferon-stimulated gene factor 3 transcriptional complex in hepatitis B virus-expressing HepG2 cells.Virol J. 2022 Feb 10;19(1):28. doi: 10.1186/s12985-022-01753-x. Virol J. 2022. PMID: 35144643 Free PMC article.
-
Suppression of USP18 Potentiates the Anti-HBV Activity of Interferon Alpha in HepG2.2.15 Cells via JAK/STAT Signaling.PLoS One. 2016 May 26;11(5):e0156496. doi: 10.1371/journal.pone.0156496. eCollection 2016. PLoS One. 2016. PMID: 27227879 Free PMC article.
-
Lipopolysaccharide and Tumor Necrosis Factor Alpha Inhibit Interferon Signaling in Hepatocytes by Increasing Ubiquitin-Like Protease 18 (USP18) Expression.J Virol. 2016 May 27;90(12):5549-5560. doi: 10.1128/JVI.02557-15. Print 2016 Jun 15. J Virol. 2016. PMID: 27009955 Free PMC article.
-
The ISG15-Protease USP18 Is a Pleiotropic Enhancer of HIV-1 Replication.Viruses. 2024 Mar 22;16(4):485. doi: 10.3390/v16040485. Viruses. 2024. PMID: 38675828 Free PMC article. Review.
-
Interferon and interferon-stimulated genes in HBV treatment.Front Immunol. 2022 Dec 1;13:1034968. doi: 10.3389/fimmu.2022.1034968. eCollection 2022. Front Immunol. 2022. PMID: 36531993 Free PMC article. Review.
Cited by
-
Roles of ubiquitin-specific proteases in inflammatory diseases.Front Immunol. 2024 Jan 23;15:1258740. doi: 10.3389/fimmu.2024.1258740. eCollection 2024. Front Immunol. 2024. PMID: 38322269 Free PMC article. Review.
-
USP18 Mediates Interferon Resistance of Dengue Virus Infection.Front Microbiol. 2021 Apr 30;12:682380. doi: 10.3389/fmicb.2021.682380. eCollection 2021. Front Microbiol. 2021. PMID: 34017322 Free PMC article.
-
Transcriptome wide functional analysis of HBx expressing human hepatocytes stimulated with endothelial cell cross-talk.Genomics. 2023 Jul;115(4):110642. doi: 10.1016/j.ygeno.2023.110642. Epub 2023 May 18. Genomics. 2023. PMID: 37209778 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

