A novel cross-species model of Barlow's disease to biomechanically analyze repair techniques in an ex vivo left heart simulator
- PMID: 32249088
- PMCID: PMC7815072
- DOI: 10.1016/j.jtcvs.2020.01.086
A novel cross-species model of Barlow's disease to biomechanically analyze repair techniques in an ex vivo left heart simulator
Abstract
Objective: Barlow's disease remains challenging to repair, given the complex valvular morphology and lack of quantitative data to compare techniques. Although there have been recent strides in ex vivo evaluation of cardiac mechanics, to our knowledge, there is no disease model that accurately simulates the morphology and pathophysiology of Barlow's disease. The purpose of this study was to design such a model.
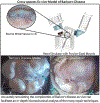
Methods: To simulate Barlow's disease, a cross-species ex vivo model was developed. Bovine mitral valves (n = 4) were sewn into a porcine annulus mount to create excess leaflet tissue and elongated chordae. A heart simulator generated physiologic conditions while hemodynamic data, high-speed videography, and chordal force measurements were collected. The regurgitant valves were repaired using nonresectional repair techniques such as neochord placement.
Results: The model successfully imitated the complexities of Barlow's disease, including redundant, billowing bileaflet tissues with notable regurgitation. After repair, hemodynamic data confirmed reduction of mitral leakage volume (25.9 ± 2.9 vs 2.1 ± 1.8 mL, P < .001) and strain gauge analysis revealed lower primary chordae forces (0.51 ± 0.17 vs 0.10 ± 0.05 N, P < .001). In addition, the maximum rate of change of force was significantly lower postrepair for both primary (30.80 ± 11.38 vs 8.59 ± 4.83 N/s, P < .001) and secondary chordae (33.52 ± 10.59 vs 19.07 ± 7.00 N/s, P = .006).
Conclusions: This study provides insight into the biomechanics of Barlow's disease, including sharply fluctuating force profiles experienced by elongated chordae prerepair, as well as restoration of primary chordae forces postrepair. Our disease model facilitates further in-depth analyses to optimize the repair of Barlow's disease.
Keywords: Barlow's disease; biomechanics; disease model; mitral regurgitation; valve repair.
Copyright © 2020 The American Association for Thoracic Surgery. Published by Elsevier Inc. All rights reserved.
Figures

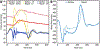


Comment in
-
Commentary: If you have to simulate, do it well!J Thorac Cardiovasc Surg. 2021 May;161(5):1786-1787. doi: 10.1016/j.jtcvs.2020.01.056. Epub 2020 Feb 8. J Thorac Cardiovasc Surg. 2021. PMID: 32139073 No abstract available.
-
Commentary: A novel cross-species model of Barlow's disease to biomechanically analyze repair techniques in an ex vivo left heart simulator.J Thorac Cardiovasc Surg. 2021 May;161(5):1784-1785. doi: 10.1016/j.jtcvs.2020.01.055. Epub 2020 Feb 8. J Thorac Cardiovasc Surg. 2021. PMID: 32143881 No abstract available.
References
Publication types
MeSH terms
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

