Microneedle array delivered recombinant coronavirus vaccines: Immunogenicity and rapid translational development
- PMID: 32249203
- PMCID: PMC7128973
- DOI: 10.1016/j.ebiom.2020.102743
Microneedle array delivered recombinant coronavirus vaccines: Immunogenicity and rapid translational development
Abstract
Background: Coronaviruses pose a serious threat to global health as evidenced by Severe Acute Respiratory Syndrome (SARS), Middle East Respiratory Syndrome (MERS), and COVID-19. SARS Coronavirus (SARS-CoV), MERS Coronavirus (MERS-CoV), and the novel coronavirus, previously dubbed 2019-nCoV, and now officially named SARS-CoV-2, are the causative agents of the SARS, MERS, and COVID-19 disease outbreaks, respectively. Safe vaccines that rapidly induce potent and long-lasting virus-specific immune responses against these infectious agents are urgently needed. The coronavirus spike (S) protein, a characteristic structural component of the viral envelope, is considered a key target for vaccines for the prevention of coronavirus infection.
Methods: We first generated codon optimized MERS-S1 subunit vaccines fused with a foldon trimerization domain to mimic the native viral structure. In variant constructs, we engineered immune stimulants (RS09 or flagellin, as TLR4 or TLR5 agonists, respectively) into this trimeric design. We comprehensively tested the pre-clinical immunogenicity of MERS-CoV vaccines in mice when delivered subcutaneously by traditional needle injection, or intracutaneously by dissolving microneedle arrays (MNAs) by evaluating virus specific IgG antibodies in the serum of vaccinated mice by ELISA and using virus neutralization assays. Driven by the urgent need for COVID-19 vaccines, we utilized this strategy to rapidly develop MNA SARS-CoV-2 subunit vaccines and tested their pre-clinical immunogenicity in vivo by exploiting our substantial experience with MNA MERS-CoV vaccines.
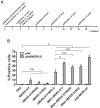
Findings: Here we describe the development of MNA delivered MERS-CoV vaccines and their pre-clinical immunogenicity. Specifically, MNA delivered MERS-S1 subunit vaccines elicited strong and long-lasting antigen-specific antibody responses. Building on our ongoing efforts to develop MERS-CoV vaccines, promising immunogenicity of MNA-delivered MERS-CoV vaccines, and our experience with MNA fabrication and delivery, including clinical trials, we rapidly designed and produced clinically-translatable MNA SARS-CoV-2 subunit vaccines within 4 weeks of the identification of the SARS-CoV-2 S1 sequence. Most importantly, these MNA delivered SARS-CoV-2 S1 subunit vaccines elicited potent antigen-specific antibody responses that were evident beginning 2 weeks after immunization.
Interpretation: MNA delivery of coronaviruses-S1 subunit vaccines is a promising immunization strategy against coronavirus infection. Progressive scientific and technological efforts enable quicker responses to emerging pandemics. Our ongoing efforts to develop MNA-MERS-S1 subunit vaccines enabled us to rapidly design and produce MNA SARS-CoV-2 subunit vaccines capable of inducing potent virus-specific antibody responses. Collectively, our results support the clinical development of MNA delivered recombinant protein subunit vaccines against SARS, MERS, COVID-19, and other emerging infectious diseases.
Keywords: COVID-19; MERS-CoV S1; Microneedle array; SARS-CoV-2; Subunit vaccines; Trimerization.
Copyright © 2020 The Author(s). Published by Elsevier B.V. All rights reserved.
Figures

References
-
- Zaki AM, van Boheemen S, Bestebroer TM, Osterhaus AD, Fouchier RA. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N Engl J Med. 2012;367(19):1814–1820. - PubMed
-
- (WHO) WHO. Middle East respiratory syndrome coronavirus (MERS-CoV). Geneva, Switzerland. Available from: http://www.who.int/emergencies/mers-cov/en/. 2018[cited 2018 November 20].
-
- Park HY, Lee EJ, Ryu YW. Epidemiological investigation of MERS-CoV spread in a single hospital in South Korea, May to June 2015. Euro Surveill Bull Eur Sur Les Malad Transm Eur Commun Disease Bull. 2015;20(25):1–6. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

