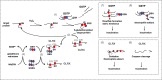
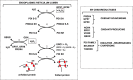
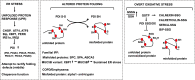
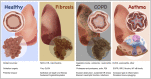
Endoplasmic reticulum stress and glutathione therapeutics in chronic lung diseases
- PMID: 32249209
- PMCID: PMC7251249
- DOI: 10.1016/j.redox.2020.101516
Endoplasmic reticulum stress and glutathione therapeutics in chronic lung diseases
Keywords: Asthma; Chronic obstructive pulmonary disease; Endoplasmic reticulum; Fibrosis; Glutaredoxin; Glutathione S-transferase P; Idiopathic pulmonary fibrosis; Mitochondria; Protein disulfide isomerase; S-glutathionylation; Unfolded protein response.
Conflict of interest statement
Declaration of competing interest Yvonne Janssen-Heininger, Niki L. Reynaert, and Vikas Anathy hold patents: United States Patent No. 8,679,811, “Treatments Involving Glutaredoxins and Similar Agents” (YJ-H, VA), United States Patent No. 8,877,447, “Detection of Glutathionylated Proteins” (YJ-H, NLR), United States Patent, 9,907,828, “Treatments of oxidative stress conditions” (YJ-H, VA) Yvonne Janssen-Heininger and Vikas Anathy have received consulting fees from Celdara Medical LLC for their contributions with the commercialization of glutaredoxin for the treatment of pulmonary fibrosis.
Figures

Similar articles
-
Glutathione S-Transferase P-Mediated Protein S-Glutathionylation of Resident Endoplasmic Reticulum Proteins Influences Sensitivity to Drug-Induced Unfolded Protein Response.Antioxid Redox Signal. 2017 Feb 20;26(6):247-261. doi: 10.1089/ars.2015.6486. Epub 2016 Mar 16. Antioxid Redox Signal. 2017. PMID: 26838680 Free PMC article.
-
Crosstalk between endoplasmic reticulum stress and oxidative stress: Focus on protein disulfide isomerase and endoplasmic reticulum oxidase 1.Eur J Pharmacol. 2021 Feb 5;892:173749. doi: 10.1016/j.ejphar.2020.173749. Epub 2020 Nov 25. Eur J Pharmacol. 2021. PMID: 33245896 Review.
-
Dysregulation of the glutaredoxin/S-glutathionylation redox axis in lung diseases.Am J Physiol Cell Physiol. 2020 Feb 1;318(2):C304-C327. doi: 10.1152/ajpcell.00410.2019. Epub 2019 Nov 6. Am J Physiol Cell Physiol. 2020. PMID: 31693398 Free PMC article.
-
Cis-element of the rice PDIL2-3 promoter is responsible for inducing the endoplasmic reticulum stress response.J Biosci Bioeng. 2014 May;117(5):620-3. doi: 10.1016/j.jbiosc.2013.10.023. Epub 2013 Dec 4. J Biosci Bioeng. 2014. PMID: 24315532
-
Protein Misfolding and Endoplasmic Reticulum Stress in Chronic Lung Disease: Will Cell-Specific Targeting Be the Key to the Cure?Chest. 2020 May;157(5):1207-1220. doi: 10.1016/j.chest.2019.11.009. Epub 2019 Nov 26. Chest. 2020. PMID: 31778676 Review.
Cited by
-
Ozone-Induced Oxidative Stress, Neutrophilic Airway Inflammation, and Glucocorticoid Resistance in Asthma.Front Immunol. 2021 Feb 26;12:631092. doi: 10.3389/fimmu.2021.631092. eCollection 2021. Front Immunol. 2021. PMID: 33717165 Free PMC article. Review.
-
GAT107-mediated α7 nicotinic acetylcholine receptor signaling attenuates inflammatory lung injury and mortality in a mouse model of ventilator-associated pneumonia by alleviating macrophage mitochondrial oxidative stress via reducing MnSOD-S-glutathionylation.Redox Biol. 2023 Apr;60:102614. doi: 10.1016/j.redox.2023.102614. Epub 2023 Jan 20. Redox Biol. 2023. PMID: 36717349 Free PMC article.
-
Effects of redox modulation on quiescin/sulfhydryl oxidase activity of melanoma cells.Mol Cell Biochem. 2024 Mar;479(3):511-524. doi: 10.1007/s11010-023-04745-9. Epub 2023 Apr 27. Mol Cell Biochem. 2024. PMID: 37103678
-
Endoplasmic Reticulum Oxidative Stress Promotes Glutathione-Dependent Oxidation of Collagen-1A1 and Promotes Lung Fibroblast Activation.Am J Respir Cell Mol Biol. 2024 Nov;71(5):589-602. doi: 10.1165/rcmb.2023-0379OC. Am J Respir Cell Mol Biol. 2024. PMID: 39042020 Free PMC article.
-
Monitoring Glutathione Content of the Endoplasmic Reticulum under Scrap Leather-Induced Endoplasmic Reticulum Stress via an Endoplasmic Reticulum-Targeted Two-Photon Fluorescent Probe.Anal Chem. 2024 Nov 12;96(45):18132-18140. doi: 10.1021/acs.analchem.4c04157. Epub 2024 Oct 29. Anal Chem. 2024. PMID: 39472451 Free PMC article.
References
-
- Mulugeta S., Nureki S., Beers M.F. Lost after translation: insights from pulmonary surfactant for understanding the role of alveolar epithelial dysfunction and cellular quality control in fibrotic lung disease. Am. J. Physiol. 2015;309(6):L507–L525. doi: 10.1152/ajplung.00139.2015. Epub 2015/07/19, PubMed PMID: 26186947; PMCID: PMC4572416. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

