A Förster Resonance Energy Transfer-Based Ratiometric Sensor with the Allosteric Transcription Factor TetR
- PMID: 32249506
- PMCID: PMC7359203
- DOI: 10.1002/smll.201907522
A Förster Resonance Energy Transfer-Based Ratiometric Sensor with the Allosteric Transcription Factor TetR
Abstract
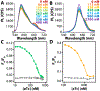
A recent description of an antibody-free assay is significantly extended for small molecule analytes using allosteric transcription factors (aTFs) and Förster resonance energy transfer (FRET). The FRET signal indicates the differential binding of an aTF-DNA pair with a dose-dependent response to its effector molecule, i.e., the analyte. The new sensors described here, based on the well-characterized aTF TetR, demonstrate several new features of the assay approach: 1) the generalizability of the sensors to additional aTF-DNA-analyte systems, 2) sensitivity modulation through the choice of donor fluorophore (quantum dots or fluorescent proteins, FPs), and 3) sensor tuning using aTF variants with differing aTF-DNA binding affinities. While all of these modular sensors self-assemble, the design reported here based on a recombinant aTF-FP chimera with commercially available dye-labeled DNA uses readily accessible sensor components to facilitate easy adoption of the sensing approach by the broader community.
Keywords: Förster resonance energy transfer (FRET); antibody-free assays; biosensors; homogeneous assays; molecular recognition; quantum dots; small molecule quantification.
© 2020 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures
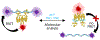


References
-
- Medintz IL, Hildebrandt N, FRET - Förster Resonance Energy Transfer: From Theory to Applications, Wiley, 2013.
-
- Wegner KD, Jin ZW, Linden S, Jennings TL, Hildebrandt N, Acs Nano 2013, 7, 7411–7419. - PubMed
-
- Wang YH, Bao L, Liu ZH, Pang DW, Anal Chem 2011, 83, 8130–8137. - PubMed
-
- Sabet FS, Hosseini M, Khabbaz H, Dadmehr M, Ganjali MR, Food Chem 2017, 220, 527–532. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

