Anomalous fractionation of mercury isotopes in the Late Archean atmosphere
- PMID: 32249783
- PMCID: PMC7136252
- DOI: 10.1038/s41467-020-15495-3
Anomalous fractionation of mercury isotopes in the Late Archean atmosphere
Abstract
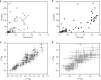
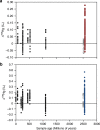
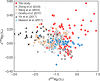
Earth's surface underwent a dramatic transition ~2.3 billion years ago when atmospheric oxygen first accumulated during the Great Oxidation Event, but the detailed composition of the reducing early atmosphere is not well known. Here we develop mercury (Hg) stable isotopes as a proxy for paleoatmospheric chemistry and use Hg isotope data from 2.5 billion-year-old sedimentary rocks to examine changes in the Late Archean atmosphere immediately prior to the Great Oxidation Event. These sediments preserve evidence of strong photochemical transformations of mercury in the absence of molecular oxygen. In addition, these geochemical records combined with previously published multi-proxy data support a vital role for methane in Earth's early atmosphere.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Anbar AD, et al. A whiff of oxygen before the great oxidation event? Geochim. Cosmochim. Acta. 2007;71:A24–A24. doi: 10.1016/j.gca.2006.10.027. - DOI
Publication types
LinkOut - more resources
Full Text Sources

