Induced osteogenic differentiation of human smooth muscle cells as a model of vascular calcification
- PMID: 32249802
- PMCID: PMC7136202
- DOI: 10.1038/s41598-020-62568-w
Induced osteogenic differentiation of human smooth muscle cells as a model of vascular calcification
Abstract
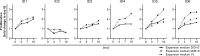
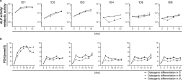
Vascular calcification is a severe pathological event in the manifestation of atherosclerosis. Pathogenic triggers mediating osteogenic differentiation of arterial smooth muscle cells (SMC) in humans remain insufficiently understood and are to a large extent investigated in animal models or cells derived thereof. Here, we describe an in vitro model based on SMC derived from healthy and diseased humans that allows to comprehensively investigate vascular calcification mechanisms. Comparing the impact of the commonly used SMC culture media VascuLife, DMEM, and M199, cells were characterised by immunofluorescence, flow cytometry, qPCR, and regarding their contractility and proliferative capacity. Irrespective of the arterial origin, the clinical background and the expansion medium used, all cells expressed typical molecular SMC marker while contractility varied between donors. Interestingly, the ability to induce an osteogenic differentiation strongly depended on the culture medium, with only SMC cultured in DMEM depositing calcified matrix upon osteogenic stimulation, which correlated with increased alkaline phosphatase activity, increased inorganic phosphate level and upregulation of osteogenic gene markers. Our optimized model is suitable for donor-oriented as well as broader screening of potential pathogenic mediators triggering vascular calcification. Translational studies aiming to identify and to evaluate therapeutic targets in a personalized fashion would be feasible.
Conflict of interest statement
The authors declare no competing interests.
Figures






Similar articles
-
Overexpression of c1q/tumor necrosis factor-related protein-3 promotes phosphate-induced vascular smooth muscle cell calcification both in vivo and in vitro.Arterioscler Thromb Vasc Biol. 2014 May;34(5):1002-10. doi: 10.1161/ATVBAHA.114.303301. Epub 2014 Feb 27. Arterioscler Thromb Vasc Biol. 2014. PMID: 24578384
-
The galectin-3/RAGE dyad modulates vascular osteogenesis in atherosclerosis.Cardiovasc Res. 2013 Dec 1;100(3):472-80. doi: 10.1093/cvr/cvt206. Epub 2013 Aug 23. Cardiovasc Res. 2013. PMID: 23975852
-
Dorsomorphin homologue 1, a highly selective small-molecule bone morphogenetic protein inhibitor, suppresses medial artery calcification.J Vasc Surg. 2017 Aug;66(2):586-593. doi: 10.1016/j.jvs.2016.03.462. Epub 2016 Jun 30. J Vasc Surg. 2017. PMID: 27374065 Free PMC article.
-
Role of smooth muscle cells in vascular calcification: implications in atherosclerosis and arterial stiffness.Cardiovasc Res. 2018 Mar 15;114(4):590-600. doi: 10.1093/cvr/cvy010. Cardiovasc Res. 2018. PMID: 29514202 Free PMC article. Review.
-
Epidemiological Research Advances in Vascular Calcification in Diabetes.J Diabetes Res. 2021 Oct 1;2021:4461311. doi: 10.1155/2021/4461311. eCollection 2021. J Diabetes Res. 2021. PMID: 34631895 Free PMC article. Review.
Cited by
-
Interleukin-29 Accelerates Vascular Calcification via JAK2/STAT3/BMP2 Signaling.J Am Heart Assoc. 2023 Jan 3;12(1):e027222. doi: 10.1161/JAHA.122.027222. Epub 2022 Dec 20. J Am Heart Assoc. 2023. PMID: 36537334 Free PMC article.
-
Vascular Calcification: Molecular Networking, Pathological Implications and Translational Opportunities.Biomolecules. 2024 Feb 25;14(3):275. doi: 10.3390/biom14030275. Biomolecules. 2024. PMID: 38540696 Free PMC article. Review.
-
The Vessels-Bone Axis: Iliac Artery Calcifications, Vertebral Fractures and Vitamin K from VIKI Study.Nutrients. 2021 Oct 12;13(10):3567. doi: 10.3390/nu13103567. Nutrients. 2021. PMID: 34684568 Free PMC article.
-
Pericentrin deficiency in smooth muscle cells augments atherosclerosis through HSF1-driven cholesterol biosynthesis and PERK activation.JCI Insight. 2023 Nov 8;8(21):e173247. doi: 10.1172/jci.insight.173247. JCI Insight. 2023. PMID: 37937642 Free PMC article.
-
Autonomous cortisol secretion promotes vascular calcification in vivo and in vitro under hyperaldosteronism.Hypertens Res. 2025 Jan;48(1):366-377. doi: 10.1038/s41440-024-01935-w. Epub 2024 Nov 8. Hypertens Res. 2025. PMID: 39516366
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

