Novel Roles of Chloroquine and Hydroxychloroquine in Graves' Orbitopathy Therapy by Targeting Orbital Fibroblasts
- PMID: 32249902
- PMCID: PMC7183395
- DOI: 10.1210/clinem/dgaa161
Novel Roles of Chloroquine and Hydroxychloroquine in Graves' Orbitopathy Therapy by Targeting Orbital Fibroblasts
Abstract
Context: Graves' orbitopathy (GO) causes infiltrative exophthalmos by inducing excessive proliferation, adipogenesis, and glycosaminoglycan production in orbital fibroblasts (OFs). Interference with OF autophagy is a potential therapy for proptosis.
Objectives: Here, we aimed to evaluate the effects of chloroquine (CQ) and hydroxychloroquine (HCQ), the autophagy inhibitors commonly used in clinical practice, on OFs.
Design/setting/participants: OFs isolated from patients with GO (GO-OFs) or control individuals (non-GO-OFs) were cultured in proliferation medium (PM) or subjected to differentiation medium. OFs were treated with CQ or HCQ (0, 0.5, 2, and 10 μM), and subsequently examined in vitro.
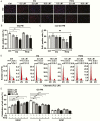
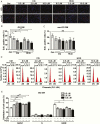
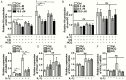
Main outcome measures: CCK-8, EdU incorporation, and flow cytometry assays were used to assess cellular viability. Adipogenesis was assessed with Western blot analysis, real-time polymerase chain reaction (PCR) , and Oil Red O staining. Hyaluronan production was determined by real-time PCR and enzyme-linked immunosorbent assay. Autophagy flux was detected through red fluorescent protein (RFP)-green fluorescent protein (GFP)-LC3 fluorescence staining and Western blot analyses.
Results: CQ/HCQ halted proliferation and adipogenesis in GO-OFs in a concentration-dependent manner through blockage of autophagy, phenotypes that were not detected in non-GO-OFs. The inhibitory effect of CQ/HCQ on hyaluronan secretion of GO-OFs was also concentration dependent, mediated by downregulation of hyaluronan synthase 2 rather than hyaluronidases. Moreover, CQ (10 μM) induced GO-OF apoptosis without aggravating oxidative stress.
Conclusions: The antimalarials CQ/HCQ affect proliferation, adipogenesis, and hyaluronan generation in GO-OFs by inhibiting autophagy, providing evidence that they can be used to treat GO as autophagy inhibitors.
Keywords: Graves’ orbitopathy; adipogenesis; autophagy; chloroquine; hydroxychloroquine; orbital fibroblasts.
© Endocrine Society 2020.
Figures


References
-
- Bartalena L, Pinchera A, Marcocci C. Management of Graves’ ophthalmopathy: reality and perspectives. Endocr Rev. 2000;21(2):168-199. - PubMed
-
- Wiersinga WM, Bartalena L. Epidemiology and prevention of Graves’ ophthalmopathy. Thyroid. 2002;12(10):855-860. - PubMed
-
- Sisti E, Coco B, Menconi F, et al. Intravenous glucocorticoid therapy for Graves’ ophthalmopathy and acute liver damage: an epidemiological study. Eur J Endocrinol. 2015;172(3):269-276. - PubMed
-
- Tanda ML, Bartalena L. Efficacy and safety of orbital radiotherapy for graves’ orbitopathy. J Clin Endocrinol Metab. 2012;97(11):3857-3865. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

