Generation of an enteric smooth muscle cell line from the pig ileum
- PMID: 32249920
- PMCID: PMC7179811
- DOI: 10.1093/jas/skaa102
Generation of an enteric smooth muscle cell line from the pig ileum
Abstract
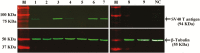
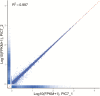
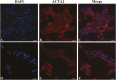
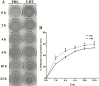
Smooth muscle cells (SMCs) play an important role in physiology and production in farm animals such as pigs. Here, we report the generation of a pig SMC line. Our original objective was to establish an enteroendocrine cell line from the pig ileum epithelium through lentiviral transduction of the Simian Virus (SV) 40 large T antigen. However, an initial expression analysis of marker genes in nine cell clones revealed that none of them were enteroendocrine cells or absorptive enterocytes, goblet cells, or Paneth cells, some of the major cell types existing in the ileum epithelium. A more detailed characterization of one clone named PIC7 by RNA-seq showed that these cells expressed many of the known smooth muscle-specific or -enriched genes, including smooth muscle actin alpha 2, calponin 1, calponin 3, myosin heavy chain 11, myosin light chain kinase, smoothelin, tenascin C, transgelin, tropomyosin 1, and tropomyosin 2. Both quantitative PCR and RNA-seq analyses showed that the PIC7 cells had a high expression of mRNA for smooth muscle actin gamma 2, also known as enteric smooth muscle actin. A Western blot analysis confirmed the expression of SV40 T antigen in the PIC7 cells. An immunohistochemical analysis demonstrated the expression of smooth muscle actin alpha 2 filaments in the PIC7 cells. A collagen gel contraction assay showed that the PIC7 cells were capable of both spontaneous contraction and contraction in response to serotonin stimulation. We conclude that the PIC7 cells are derived from an enteric SMC from the pig ileum. These cells may be a useful model for studying the cellular and molecular physiology of pig enteric SMCs. Because pigs are similar to humans in anatomy and physiology, the PIC7 cells may be also used as a model for human intestinal SMCs.
Keywords: epithelium; ileum; pigs; small intestine; smooth muscle.
© The Author(s) 2020. Published by Oxford University Press on behalf of the American Society of Animal Science. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures

Similar articles
-
Similarities and differences in smooth muscle alpha-actin induction by TGF-beta in smooth muscle versus non-smooth muscle cells.Arterioscler Thromb Vasc Biol. 1999 Sep;19(9):2049-58. doi: 10.1161/01.atv.19.9.2049. Arterioscler Thromb Vasc Biol. 1999. PMID: 10479645
-
Changes in Ca2+ signaling and contractile protein isoforms in smooth muscle cells from guinea pig ileum during culture.J Smooth Muscle Res. 2001 Apr;37(2):53-66. doi: 10.1540/jsmr.37.53. J Smooth Muscle Res. 2001. PMID: 11592284
-
Smooth Muscle-Like Cells Generated from Human Mesenchymal Stromal Cells Display Marker Gene Expression and Electrophysiological Competence Comparable to Bladder Smooth Muscle Cells.PLoS One. 2015 Dec 16;10(12):e0145153. doi: 10.1371/journal.pone.0145153. eCollection 2015. PLoS One. 2015. PMID: 26673782 Free PMC article.
-
Calponin.Int J Biochem Cell Biol. 1996 Nov;28(11):1185-9. doi: 10.1016/s1357-2725(96)00085-4. Int J Biochem Cell Biol. 1996. PMID: 9022277 Review.
-
Regulation of smooth muscle actin-myosin interaction and force by calponin.Acta Physiol Scand. 1998 Dec;164(4):415-26. doi: 10.1111/j.1365-201x.1998.tb10697.x. Acta Physiol Scand. 1998. PMID: 9887965 Review.
Cited by
-
Porcine intestinal organoids cultured in an organ-on-a-chip microphysiological system.Biochem Biophys Rep. 2025 May 4;42:102036. doi: 10.1016/j.bbrep.2025.102036. eCollection 2025 Jun. Biochem Biophys Rep. 2025. PMID: 40421277 Free PMC article.
-
Establishment of a bovine rumen epithelial cell line.J Anim Sci. 2021 Oct 1;99(10):skab273. doi: 10.1093/jas/skab273. J Anim Sci. 2021. PMID: 34570883 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

