Epicutaneous sensitization in the development of food allergy: What is the evidence and how can this be prevented?
- PMID: 32249942
- PMCID: PMC7494573
- DOI: 10.1111/all.14304
Epicutaneous sensitization in the development of food allergy: What is the evidence and how can this be prevented?
Abstract
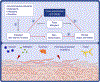
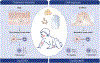
There is increasing evidence regarding the importance of allergic sensitization through the skin. In this review, we provide an overview of the atopic march and immune mechanism underlying the sensitization and effector phase of food allergy. We present experimental models and human data that support the concept of epicutaneous sensitization and how this forms one half of the dual-allergen exposure hypothesis. We discuss specific important elements in the skin (FLG and other skin barrier gene mutations, Langerhans cells, type 2 innate lymphoid cells, IL-33, TSLP) that have important roles in the development of allergic responses as well as the body of evidence on environmental allergen exposure and how this can sensitize an individual. Given the link between skin barrier impairment, atopic dermatitis, food allergy, allergic asthma, and allergic rhinitis, it is logical that restoring the skin barrier and prevention or treating atopic dermatitis would have beneficial effects on prevention of related allergic diseases, particularly food allergy. We present the experimental and human studies that have evaluated this approach and discuss various factors which may influence the success of these approaches, such as the type of emollient chosen for the intervention, the role of managing skin inflammation, and differences between primary and secondary prevention of atopic dermatitis to achieve the desired outcome.
Keywords: atopic dermatitis; cutaneous sensitization; emollient; filaggrin gene; food allergy.
© 2020 The Authors. Allergy published by European Academy of Allergy and Clinical Immunology and John Wiley & Sons Ltd.
Conflict of interest statement
Conflict of Interest Statement
Dr. Brough reports personal fees from DBV Technologies, and non-financial support from ThermoScientific; Dr. Nadeau reports grants from National Institute of Allergy and Infectious Diseases (NIAID), Food Allergy Research & Education (FARE), End Allergies Together (EAT), Allergenis, and Ukko Pharma; Grant awardee at NIAID, National Institute of Environmental Health Sciences (NIEHS), National Heart, Lung, and Blood Institute (NHLBI), and the Environmental Protection Agency (EPA); is involved in Clinical trials with Regeneron, Genentech, AImmune Therapeutics, DBV Technologies, AnaptysBio, Adare Pharmaceuticals, and Stallergenes-Greer; Research Sponsorship by Novartis, Sanofi, Astellas, Nestle; Data and Safety Monitoring Board member at Novartis and NHLBI; Cofounded Before Brands, Alladapt, ForTra, and Iggenix; Chief Intellectual Office at FARE, Director of the World Allergy Organization (WAO) Center of Excellence at Stanford, Personal fees from Regeneron, Astrazeneca, ImmuneWorks, and Cour Pharmaceuticals; Consultant and Advisory Board Member at Ukko, Before Brands, Alladapt, IgGenix, Probio, Vedanta, Centecor, Seed, Novartis, NHBLI, EPA, National Scientific Committee of ITN and NIH Programs; US patents (patent numbers 62/647,389; 62/119,014; 12/610,940, 12/686,121, 10/064,936, 62/767,444; application numbers S10–392); Dr. Sindher reports grants from Aimmune, DBV Technologies, and Regeneron; Dr. Chan reports non-financial support from Novartis and grants from Aimmune; Dr. Bahnson reports personal fees from King’s College and DBV technologies; Dr. Lack reports grants from National Institute of Allergy and Infectious
Diseases (NIAID, NIH), Food Allergy & Research Education (FARE), MRC & Asthma UK Centre, UK Dept of Health through NIHR, National Peanut Board (NPB), UK Food Standards Agency (FSA), The Davis Foundation, Scientific Advisory Board member and Stock Options at DBV Technologies, Shareholder at Mighty Mission Me, personal fees/consultancy from Novartis, personal fees/consultancy from Sanofi-Genyzme, personal fees/consultancy from Regeneron, personal fees and consultancy from ALK-Abello; Drs. Alkotob and Leung have nothing to disclose
Figures




References
-
- Martin PE, Koplin JJ, Eckert JK, Lowe AJ, Ponsonby AL, Osborne NJ, et al. The prevalence and socio-demographic risk factors of clinical eczema in infancy: a population-based observational study. Clin Exp Allergy 2013;43:642–651. - PubMed
-
- Wollenberg A, Barbarot S, Bieber T, Christen-Zaech S, Deleuran M, Fink-Wagner A, et al. Consensus-based European guidelines for treatment of atopic eczema (atopic dermatitis) in adults and children: part I. J Eur Acad Dermatol Venereol 2018;32:657–682. - PubMed
-
- Borna E, Nwaru BI, Bjerg A, Mincheva R, Radinger M, Lundback B, et al. Changes in the prevalence of asthma and respiratory symptoms in western Sweden between 2008 and 2016. Allergy 2019;74:1703–1715. - PubMed
-
- Global Initiative for Asthma. Global strategy for asthma management and prevention (2018 update). 2018. Available from: https://ginasthma.org/wp-content/uploads/2019/06/GINA-2019-main-report-J.... Accessed Feb 24, 2020
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

