Analysis of 61 exclusive enteral nutrition formulas used in the management of active Crohn's disease-new insights into dietary disease triggers
- PMID: 32249975
- PMCID: PMC8653890
- DOI: 10.1111/apt.15695
Analysis of 61 exclusive enteral nutrition formulas used in the management of active Crohn's disease-new insights into dietary disease triggers
Abstract
Background: Exclusive enteral nutrition (EEN) is an effective treatment for Crohn's disease.
Aims: To investigate the hypothesis that ingredients of EEN formulas are unlikely to initiate a disease flare and that their dietary elimination is not essential for disease amelioration.
Methods: We performed compositional analysis of EEN formulas with evidence of efficacy in management of active Crohn's disease. Macronutrient content was compared against the dietary reference values (DRV), the UK National Diet and Nutrition Survey (NDNS) and intake of Crohn's disease children. Food additives were cross-referenced against the FAO/WHO database.
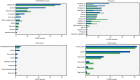
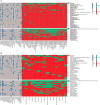
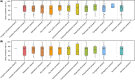
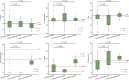
Results: Sixty-one formulas were identified with variable composition (carbohydrates [22.8%-89.3%], protein [7.8%-30.1%], fat [0%-52.5%]). Maltodextrin, milk protein and vegetable/plant oils were the commonest macronutrient sources. Their n-6:n-3 fatty acid ratio varied from 0.25 to 46.5. 56 food additives were identified (median per formula: 11). All formulas were lactose-free, gluten-free, and 82% lacked fibre. The commonest food additives were emulsifiers, stabilisers, antioxidants, acidity regulators and thickeners. Food additives, implicated in Crohn's disease aetiology, were present in formulas (modified starches [100%], carrageenan [22%], carboxymethyl cellulose [13%] and polysorbate 80 [5%]). Remission rates did not differ between EEN formulas with and without those food additives. Analysis including only formulas from randomised controlled trials (RCTs) retained in the latest Cochrane meta-analysis produced similar findings. EEN formulas contained less energy from saturated fat than NDNS intake.
Conclusion: We have identified food ingredients which are present in EEN formulas that are effective in Crohn's disease and challenge perceptions that these ingredients might be harmful.
© 2020 The Authors. Alimentary Pharmacology & Therapeutics published by John Wiley & Sons Ltd.
Figures
References
-
- Ng SC, Shi HY, Hamidi N, et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population‐based studies. Lancet (London, England). 2018;390:2769‐2778. - PubMed
-
- Hou JK, Abraham B, El‐Serag H. Dietary intake and risk of developing inflammatory bowel disease: a systematic review of the literature. Am J Gastroenterol. 2011;106:563‐573. - PubMed
-
- Wedlake L, Slack N, Andreyev HJ, Whelan K. Fiber in the treatment and maintenance of inflammatory bowel disease: a systematic review of randomized controlled trials. Inflamm Bowel Dis. 2014;20:576‐586. - PubMed
