Ligand-Enabled γ-C(sp3 )-H Olefination of Free Carboxylic Acids
- PMID: 32250014
- PMCID: PMC7496353
- DOI: 10.1002/anie.202002362
Ligand-Enabled γ-C(sp3 )-H Olefination of Free Carboxylic Acids
Abstract
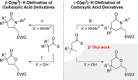
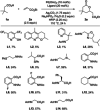
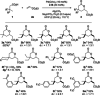
We report the ligand-enabled C-H activation/olefination of free carboxylic acids in the γ-position. Through an intramolecular Michael addition, δ-lactones are obtained as products. Two distinct ligand classes are identified that enable the challenging palladium-catalyzed activation of free carboxylic acids in the γ-position. The developed protocol features a wide range of acid substrates and olefin reaction partners and is shown to be applicable on a preparatively useful scale. Insights into the underlying reaction mechanism obtained through kinetic studies are reported.
Keywords: C−H activation; carboxylic acids; ligand-enabled catalysis; palladium; δ-lactones.
© 2020 The Authors. Published by Wiley-VCH Verlag GmbH & Co. KGaA.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Distal γ-C(sp3 )-H Olefination of Ketone Derivatives and Free Carboxylic Acids.Angew Chem Int Ed Engl. 2020 Jul 27;59(31):12853-12859. doi: 10.1002/anie.202003271. Epub 2020 Jun 5. Angew Chem Int Ed Engl. 2020. PMID: 32385966 Free PMC article.
-
Pd(II)-Catalyzed Synthesis of Bicyclo[3.2.1] Lactones via Tandem Intramolecular β-C(sp3)-H Olefination and Lactonization of Free Carboxylic Acids.J Am Chem Soc. 2022 Jul 13;144(27):11955-11960. doi: 10.1021/jacs.2c04195. Epub 2022 Jun 28. J Am Chem Soc. 2022. PMID: 35763801 Free PMC article.
-
One-Step Synthesis of β-Alkylidene-γ-lactones via Ligand-Enabled β,γ-Dehydrogenation of Aliphatic Acids.J Am Chem Soc. 2022 Jul 20;144(28):12924-12933. doi: 10.1021/jacs.2c04779. Epub 2022 Jul 8. J Am Chem Soc. 2022. PMID: 35802794 Free PMC article.
-
Ligand-Enabled β-C(sp3)-H Olefination of Free Carboxylic Acids.J Am Chem Soc. 2018 Aug 15;140(32):10363-10367. doi: 10.1021/jacs.8b06527. Epub 2018 Aug 2. J Am Chem Soc. 2018. PMID: 30029574 Free PMC article.
-
The Emergence of Palladium-Catalyzed C(sp3 )-H Functionalization of Free Carboxylic Acids.Chem Asian J. 2021 Mar 1;16(5):397-408. doi: 10.1002/asia.202001440. Epub 2021 Feb 2. Chem Asian J. 2021. PMID: 33427411 Review.
Cited by
-
Transition-Metal-Catalyzed, Coordination-Assisted Functionalization of Nonactivated C(sp3)-H Bonds.Chem Rev. 2021 Dec 22;121(24):14957-15074. doi: 10.1021/acs.chemrev.1c00519. Epub 2021 Oct 29. Chem Rev. 2021. PMID: 34714620 Free PMC article. Review.
-
Access to unsaturated bicyclic lactones by overriding conventional C(sp3)-H site selectivity.Nat Chem. 2023 Nov;15(11):1626-1635. doi: 10.1038/s41557-023-01295-x. Epub 2023 Aug 10. Nat Chem. 2023. PMID: 37563324 Free PMC article.
-
Synthesis of γ-alkylidene lactones via molecular stitching of carboxylic acids and olefins.Chem Sci. 2025 Jul 4;16(32):14578-14583. doi: 10.1039/d5sc03349g. eCollection 2025 Aug 13. Chem Sci. 2025. PMID: 40666195 Free PMC article.
-
Catalyst-Controlled Chemoselective γ-C(sp3)-H Lactonization of Carboxylic Acid: Methyl versus Methylene.J Am Chem Soc. 2024 Oct 30;146(43):29311-29314. doi: 10.1021/jacs.4c12907. Epub 2024 Oct 16. J Am Chem Soc. 2024. PMID: 39412381 Free PMC article.
-
Recent development in transition metal-catalysed C-H olefination.Chem Sci. 2021 Jan 29;12(8):2735-2759. doi: 10.1039/d0sc05555g. Chem Sci. 2021. PMID: 34164039 Free PMC article. Review.
References
Publication types
LinkOut - more resources
Full Text Sources

