Ligand-Enabled γ-C(sp3 )-H Olefination of Free Carboxylic Acids
- PMID: 32250014
- PMCID: PMC7496353
- DOI: 10.1002/anie.202002362
Ligand-Enabled γ-C(sp3 )-H Olefination of Free Carboxylic Acids
Abstract
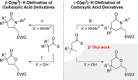
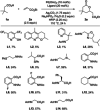
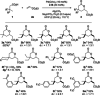
We report the ligand-enabled C-H activation/olefination of free carboxylic acids in the γ-position. Through an intramolecular Michael addition, δ-lactones are obtained as products. Two distinct ligand classes are identified that enable the challenging palladium-catalyzed activation of free carboxylic acids in the γ-position. The developed protocol features a wide range of acid substrates and olefin reaction partners and is shown to be applicable on a preparatively useful scale. Insights into the underlying reaction mechanism obtained through kinetic studies are reported.
Keywords: C−H activation; carboxylic acids; ligand-enabled catalysis; palladium; δ-lactones.
© 2020 The Authors. Published by Wiley-VCH Verlag GmbH & Co. KGaA.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
Publication types
LinkOut - more resources
Full Text Sources

