Association of Factors With Elevated Amyloid Burden in Clinically Normal Older Individuals
- PMID: 32250387
- PMCID: PMC7136861
- DOI: 10.1001/jamaneurol.2020.0387
Association of Factors With Elevated Amyloid Burden in Clinically Normal Older Individuals
Abstract
Importance: The Anti-Amyloid Treatment in Asymptomatic Alzheimer disease (A4) Study is an ongoing prevention trial in clinically normal older individuals with evidence of elevated brain amyloid. The large number of participants screened with amyloid positron emission tomography (PET) and standardized assessments provides an unprecedented opportunity to evaluate factors associated with elevated brain amyloid.
Objective: To investigate the association of elevated amyloid with demographic and lifestyle factors, apolipoprotein E (APOE), neuropsychological testing, and self- and study partner reports of cognitive function.
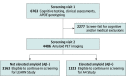
Design, setting, and participants: This cross-sectional study included screening data in the Anti-Amyloid Treatment in Asymptomatic Alzheimer Disease (A4) Study collected from April 2014 to December 2017 and classified by amyloid status. Data were was analyzed from 2018 to 2019 across 67 sites in the US, Canada, Australia, and Japan and included 4486 older individuals (age 65-85 years) who were eligible for amyloid PET (clinically normal [Clinical Dementia Rating = 0] and cognitively unimpaired [Mini-Mental State Examination score, ≥25; logical memory IIa 6-18]).
Main outcomes and measures: Screening demographics, lifestyle variables, APOE genotyping, and cognitive testing (Preclinical Alzheimer Cognitive Composite), self- and study partner reports of high-level daily cognitive function (Cognitive Function Index). Florbetapir amyloid PET imaging was used to classify participants as having elevated amyloid (Aβ+) or not having elevated amyloid (Aβ-).
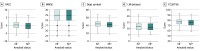
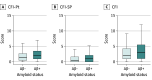
Results: Amyloid PET results were acquired for 4486 participants (mean [SD] age, 71.29 [4.67] years; 2647 women [59%]), with 1323 (29.5%) classified as Aβ+. Aβ+ participants were slightly older than Aβ-, with no observed differences in sex, education, marital or retirement status, or any self-reported lifestyle factors. Aβ+ participants were more likely to have a family history of dementia (3320 Aβ+ [74%] vs 3050 Aβ- [68%]) and at least 1 APOE ε4 allele (2602 Aβ+ [58%] vs 1122 Aβ- [25%]). Aβ+ participants demonstrated worse performance on screening Preclinical Alzheimer Cognitive Composite results and reported higher change scores on the Cognitive Function Index.
Conclusions and relevance: Among a large group of older individuals screening for an Alzheimer disease (AD) prevention trial, elevated brain amyloid was associated with family history and APOE ε4 allele but not with multiple other previously reported risk factors for AD. Elevated amyloid was associated with lower test performance results and increased reports of subtle recent declines in daily cognitive function. These results support the hypothesis that elevated amyloid represents an early stage in the Alzheimer continuum and demonstrate the feasibility of enrolling these high-risk participants in secondary prevention trials aimed at slowing cognitive decline during the preclinical stages of AD.
Conflict of interest statement
Figures
References
-
- Sperling RA, Aisen PS, Beckett LA, et al. . Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):280-292. doi:10.1016/j.jalz.2011.03.003 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

