Sample Pooling as a Strategy to Detect Community Transmission of SARS-CoV-2
- PMID: 32250394
- PMCID: PMC7136853
- DOI: 10.1001/jama.2020.5445
Sample Pooling as a Strategy to Detect Community Transmission of SARS-CoV-2
Abstract
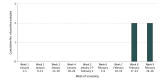
This study describes findings of novel coronavirus testing on pooled nasopharyngeal and bronchoalveolar lavage samples taken from patients who had negative results by routine respiratory virus testing to see if pooling samples could increase testing throughput and efficiency and facilitate early detection of community COVID-19 transmission.
Conflict of interest statement
Figures

References
-
- Centers for Disease Control and Prevention Evaluating and testing persons for coronavirus disease 2019 (COVID-19). Updated March 24, 2020. Accessed March 25 2020. https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-criteria.html
-
- Santa Clara County Public Health Healthy Santa Clara County: novel coronavirus (COVID-19) data dashboard. Accessed March 25, 2020. https://www.sccgov.org/sites/phd/DiseaseInformation/novel-coronavirus/Pa...
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

