Evolution of new enzymes by gene duplication and divergence
- PMID: 32250558
- PMCID: PMC9306413
- DOI: 10.1111/febs.15299
Evolution of new enzymes by gene duplication and divergence
Abstract
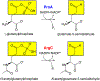
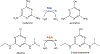
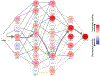
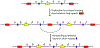
Thousands of new metabolic and regulatory enzymes have evolved by gene duplication and divergence since the dawn of life. New enzyme activities often originate from promiscuous secondary activities that have become important for fitness due to a change in the environment or a mutation. Mutations that make a promiscuous activity physiologically relevant can occur in the gene encoding the promiscuous enzyme itself, but can also occur elsewhere, resulting in increased expression of the enzyme or decreased competition between the native and novel substrates for the active site. If a newly useful activity is inefficient, gene duplication/amplification will set the stage for divergence of a new enzyme. Even a few mutations can increase the efficiency of a new activity by orders of magnitude. As efficiency increases, amplified gene arrays will shrink to provide two alleles, one encoding the original enzyme and one encoding the new enzyme. Ultimately, genomic rearrangements eliminate co-amplified genes and move newly evolved paralogs to a distant region of the genome.
Keywords: Innovation-amplification-divergence model; directed evolution; enzyme evolution; gene duplication; promiscuity.
© 2020 Federation of European Biochemical Societies.
Conflict of interest statement
Conflicts of interest
None.
Figures














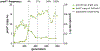
References
-
- Zhou Y, Minio A, Massonnet M, Solares E, Lv Y, Beridze T, Cantu D & Gaut BS (2019) The population genetics of structural variants in grapevine domestication, Nat Plants. 5, 965–979. - PubMed
-
- Ohno S (1970) Evolution by gene duplication., Springer-Verlag, New York.
-
- Wolfe KH & Shields DC (1997) Molecular evidence for an ancient duplication of the entire yeast genome, Nature. 387, 708–13. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

