Neuromodulation of Brain State and Behavior
- PMID: 32250724
- PMCID: PMC12237593
- DOI: 10.1146/annurev-neuro-100219-105424
Neuromodulation of Brain State and Behavior
Abstract
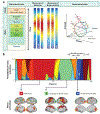
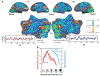
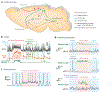
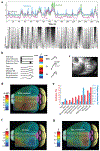
Neural activity and behavior are both notoriously variable, with responses differing widely between repeated presentation of identical stimuli or trials. Recent results in humans and animals reveal that these variations are not random in their nature, but may in fact be due in large part to rapid shifts in neural, cognitive, and behavioral states. Here we review recent advances in the understanding of rapid variations in the waking state, how variations are generated, and how they modulate neural and behavioral responses in both mice and humans. We propose that the brain has an identifiable set of states through which it wanders continuously in a nonrandom fashion, owing to the activity of both ascending modulatory and fast-acting corticocortical and subcortical-cortical neural pathways. These state variations provide the backdrop upon which the brain operates, and understanding them is critical to making progress in revealing the neural mechanisms underlying cognition and behavior.
Keywords: cerebral cortex; human; perception; performance; spontaneous activity; variability.
Figures

References
-
- Aston-Jones G, Cohen JD. 2005. An integrative theory of locus coeruleus-norepinephrine function: adaptive gain and optimal performance. Annu. Rev. Neurosci. 28:403–50 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

