Biomarkers for Alzheimer's disease-preparing for a new era of disease-modifying therapies
- PMID: 32251378
- PMCID: PMC8172244
- DOI: 10.1038/s41380-020-0721-9
Biomarkers for Alzheimer's disease-preparing for a new era of disease-modifying therapies
Abstract
Clinical trial results presented in 2019 suggest that antibody-based removal of cerebral amyloid β (Aβ) plaques may possibly clear tau tangles and modestly slow cognitive decline in symptomatic Alzheimer's disease (AD). Although regulatory approval of this approach is still pending, preparing the healthcare system for the advent of disease-modifying therapies against AD is imperative. In particular, it will be necessary to identify the most suitable biomarkers to facilitate appropriate treatment of AD. Here, we give an update on recent developments in fluid and imaging biomarkers for AD-related pathologies and discuss potential approaches that could be adopted to screen for and clarify the underlying pathology in people seeking medical advice because of cognitive symptoms. We succinctly review recent data regarding biomarkers for Aβ and tau pathology, neurodegeneration, synaptic dysfunction, and inflammation, highlight the need for further research into common copathologies, and suggest how different biomarkers could be used (most likely in combination) to facilitate the development and clinical implementation of novel drug candidates against AD.
Conflict of interest statement
Conflicts of interest
HZ has served at scientific advisory boards for Denali, Roche Diagnostics, Wave, Samumed and CogRx, has given lectures in symposia sponsored by Fujirebio, Alzecure and Biogen, and is a co-founder of Brain Biomarker Solutions in Gothenburg AB, a GU Ventures-based platform company at the University of Gothenburg. BBB has received precursors and imaging agents from Avid Radiopharmaceuticals.
Figures
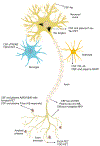
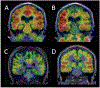
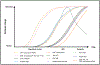
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

