Engineered reproductive tissues
- PMID: 32251392
- PMCID: PMC7416444
- DOI: 10.1038/s41551-020-0525-x
Engineered reproductive tissues
Erratum in
-
Author Correction: Engineered reproductive tissues.Nat Biomed Eng. 2020 May;4(5):574. doi: 10.1038/s41551-020-0561-6. Nat Biomed Eng. 2020. PMID: 32332996
Abstract
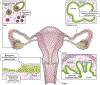
Engineered male and female biomimetic reproductive tissues are being developed as autonomous in vitro units or as integrated multi-organ in vitro systems to support germ cell and embryo function, and to display characteristic endocrine phenotypic patterns, such as the 28-day human ovulatory cycle. In this Review, we summarize how engineered reproductive tissues facilitate research in reproductive biology, and overview strategies for making engineered reproductive tissues that might eventually allow the restoration of reproductive capacity in patients.
Figures
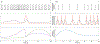
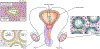

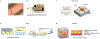
References
-
- Morris RT The ovarian graft. New York Med. J 62, 436 (1895).
-
- Favre-Inhofer A, Rafii A, Carbonnel M, Revaux A & Ayoubi JM Uterine transplantation: review in human research. J. Gynecol. Obstet. Hum. Reprod 47, 213–221 (2018). - PubMed
-
- Girsdansky J & Newman HF Use of a vitallium testicular implant. Am. J. Surg 53, 514 (1941)
-
- Steptoe PC & Edwards RG Birth after the reimplantation of a human embryo. Lancet 312, 366 (1978) - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

