Biochemical Characterization of Yeast Xrn1
- PMID: 32251580
- PMCID: PMC7780157
- DOI: 10.1021/acs.biochem.9b01035
Biochemical Characterization of Yeast Xrn1
Abstract
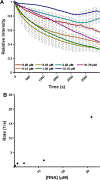
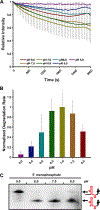
Messenger RNA degradation is an important component of overall gene expression. During the final step of eukaryotic mRNA degradation, exoribonuclease 1 (Xrn1) carries out 5' → 3' processive, hydrolytic degradation of RNA molecules using divalent metal ion catalysis. To initiate studies of the 5' → 3' RNA decay machinery in our lab, we expressed a C-terminally truncated version of Saccharomyces cerevisiae Xrn1 and explored its enzymology using a second-generation, time-resolved fluorescence RNA degradation assay. Using this system, we quantitatively explored Xrn1's preference for 5'-monophosphorylated RNA substrates, its pH dependence, and the importance of active site mutations in the molecule's conserved catalytic core. Furthermore, we explore Xrn1's preference for RNAs containing a 5' single-stranded region both in an intermolecular hairpin structure and in an RNA-DNA hybrid duplex system. These results both expand and solidify our understanding of Xrn1, a centrally important enzyme whose biochemical properties have implications in numerous RNA degradation and processing pathways.
Conflict of interest statement
The authors declare no competing financial interest.
Figures







References
-
- Parker R, and Song H (2004) The enzymes and control of eukaryotic mRNA turnover. Nat. Struct. Mol. Biol. 11, 121–127. - PubMed
-
- Garneau NL, Wilusz J, and Wilusz CJ (2007) The highways and byways of mRNA decay. Nat. Rev. Mol. Cell Biol. 8, 113–126. - PubMed
-
- Lebreton A, Tomecki R, Dziembowski A, and Séraphin B (2008) Endonucleolytic RNA cleavage by a eukaryotic exosome. Nature 456, 993–996. - PubMed
-
- Fabian MR, Sonenberg N, and Filipowicz W (2010) Regulation of mRNA Translation and Stability by microRNAs. Annu. Rev. Biochem. 79, 351–379. - PubMed
-
- Larimer FW, Hsu CL, Maupin MK, and Stevens A (1992) Characterization of the XRN1 gene encoding a 5′→3′ exoribonuclease: sequence data and analysis of disparate protein and mRNA levels of gene-disrupted yeast cells. Gene 120, 51–57. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

