Safety and immunogenicity of a parenteral trivalent P2-VP8 subunit rotavirus vaccine: a multisite, randomised, double-blind, placebo-controlled trial
- PMID: 32251641
- PMCID: PMC7322558
- DOI: 10.1016/S1473-3099(20)30001-3
Safety and immunogenicity of a parenteral trivalent P2-VP8 subunit rotavirus vaccine: a multisite, randomised, double-blind, placebo-controlled trial
Abstract
Background: A monovalent, parenteral, subunit rotavirus vaccine was well tolerated and immunogenic in adults in the USA and in toddlers and infants in South Africa, but elicited poor responses against heterotypic rotavirus strains. We aimed to evaluate safety and immunogenicity of a trivalent vaccine formulation (P2-VP8-P[4],[6],[8]).
Methods: A double-blind, randomised, placebo-controlled, dose-escalation, phase 1/2 study was done at three South African research sites. Healthy adults (aged 18-45 years), toddlers (aged 2-3 years), and infants (aged 6-8 weeks, ≥37 weeks' gestation, and without previous receipt of rotavirus vaccination), all without HIV infection, were eligible for enrolment. In the dose-escalation phase, adults and toddlers were randomly assigned in blocks (block size of five) to receive 30 μg or 90 μg of vaccine, or placebo, and infants were randomly assigned in blocks (block size of four) to receive 15 μg, 30 μg, or 90 μg of vaccine, or placebo. In the expanded phase, infants were randomly assigned in a 1:1:1:1 ratio to receive 15 μg, 30 μg, or 90 μg of vaccine, or placebo, in block sizes of four. Participants, parents of participants, and clinical, data, and laboratory staff were masked to treatment assignment. Adults received an intramuscular injection of vaccine or placebo in the deltoid muscle on the day of randomisation (day 0), day 28, and day 56; toddlers received a single injection of vaccine or placebo in the anterolateral thigh on day 0. Infants in both phases received an injection of vaccine or placebo in the anterolateral thigh on days 0, 28, and 56, at approximately 6, 10, and 14 weeks of age. Primary safety endpoints were local and systemic reactions (grade 2 or worse) within 7 days and adverse events and serious adverse events within 28 days after each injection in all participants who received at least one injection. Primary immunogenicity endpoints were analysed in infants in either phase who received all planned injections, had blood samples analysed at the relevant timepoints, and presented no major protocol violations considered to have an effect on the immunogenicity results of the study, and included serum anti-P2-VP8 IgA, IgG, and neutralising antibody geometric mean titres and responses measured 4 weeks after the final injection in vaccine compared with placebo groups. This trial is registered with ClinicalTrials.gov, NCT02646891.
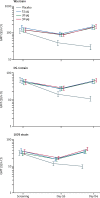
Findings: Between Feb 15, 2016, and Dec 22, 2017, 30 adults (12 each in the 30 μg and 90 μg groups and six in the placebo group), 30 toddlers (12 each in the 30 μg and 90 μg groups and six in the placebo group), and 557 infants (139 in the 15 μg group, 140 in the 30 μg group, 139 in the 90 μg group, and 139 in the placebo group) were randomly assigned, received at least one dose, and were assessed for safety. There were no significant differences in local or systemic adverse events, or unsolicited adverse events, between vaccine and placebo groups. There were no serious adverse events within 28 days of injection in adults, whereas one serious adverse event occurred in a toddler (febrile convulsion in the 30 μg group) and 23 serious adverse events (four in placebo, ten in 15 μg, four in 30 μg, and five in 90 μg groups) occurred among 20 infants, most commonly respiratory tract infections. One death occurred in an infant within 28 days of injection due to pneumococcal meningitis. In 528 infants (130 in placebo, 132 in 15 μg, 132 in 30 μg, and 134 in 90 μg groups), adjusted anti-P2-VP8 IgG seroresponses (≥4-fold increase from baseline) to P[4], P[6], and P[8] antigens were significantly higher in the 15 μg, 30 μg, and 90 μg groups (99-100%) than in the placebo group (10-29%; p<0·0001). Although significantly higher than in placebo recipients (9-10%), anti-P2-VP8 IgA seroresponses (≥4-fold increase from baseline) to each individual antigen were modest (20-34%) across the 15 μg, 30 μg, and 90 μg groups. Adjusted neutralising antibody seroresponses in infants (≥2·7-fold increase from baseline) to DS-1 (P[4]), 1076 (P[6]), and Wa (P[8]) were higher in vaccine recipients than in placebo recipients: p<0·0001 for all comparisons.
Interpretation: The trivalent P2-VP8 vaccine was well tolerated, with promising anti-P2-VP8 IgG and neutralising antibody responses across the three vaccine P types. Our findings support advancing the vaccine to efficacy testing.
Funding: Bill & Melinda Gates Foundation.
Copyright © 2020 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 license. Published by Elsevier Ltd.. All rights reserved.
Figures

References
-
- Jonesteller CL, Burnett E, Yen C, Tate JE, Parashar UD. Effectiveness of rotavirus vaccination: a systematic review of the first decade of global postlicensure data, 2006–2016. Clin Infect Dis. 2017;65:840–850. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

