Structural and molecular modelling studies reveal a new mechanism of action of chloroquine and hydroxychloroquine against SARS-CoV-2 infection
- PMID: 32251731
- PMCID: PMC7128678
- DOI: 10.1016/j.ijantimicag.2020.105960
Structural and molecular modelling studies reveal a new mechanism of action of chloroquine and hydroxychloroquine against SARS-CoV-2 infection
Abstract
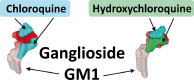
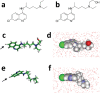
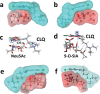
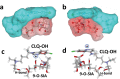
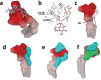
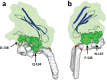
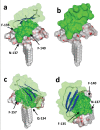
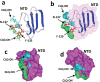
The recent emergence of the novel pathogenic SARS-coronavirus 2 (SARS-CoV-2) is responsible for a worldwide pandemic. Given the global health emergency, drug repositioning is the most reliable option to design an efficient therapy for infected patients without delay. The first step of the viral replication cycle [i.e. attachment to the surface of respiratory cells, mediated by the spike (S) viral protein] offers several potential therapeutic targets. The S protein uses the angiotension-converting enzyme-2 (ACE-2) receptor for entry, but also sialic acids linked to host cell surface gangliosides. Using a combination of structural and molecular modelling approaches, this study showed that chloroquine (CLQ), one of the drugs currently under investigation for SARS-CoV-2 treatment, binds sialic acids and gangliosides with high affinity. A new type of ganglioside-binding domain at the tip of the N-terminal domain of the SARS-CoV-2 S protein was identified. This domain (111-158), which is fully conserved among clinical isolates worldwide, may improve attachment of the virus to lipid rafts and facilitate contact with the ACE-2 receptor. This study showed that, in the presence of CLQ [or its more active derivative, hydroxychloroquine (CLQ-OH)], the viral S protein is no longer able to bind gangliosides. The identification of this new mechanism of action of CLQ and CLQ-OH supports the use of these repositioned drugs to cure patients infected with SARS-CoV-2. The in-silico approaches used in this study might also be used to assess the efficiency of a broad range of repositioned and/or innovative drug candidates before clinical evaluation.
Keywords: Chloroquine; Coronavirus; Ganglioside; Pandemic; SARS-CoV-2; Spike.
Copyright © 2020 Elsevier B.V. and International Society of Chemotherapy. All rights reserved.
Figures


References
-
- Mullard A. Drug repurposing programmes get lift off. Nat Rev Drug Discov. 2012;11:505–506. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

