Engineered Human Cathelicidin Antimicrobial Peptides Inhibit Ebola Virus Infection
- PMID: 32252021
- PMCID: PMC7104201
- DOI: 10.1016/j.isci.2020.100999
Engineered Human Cathelicidin Antimicrobial Peptides Inhibit Ebola Virus Infection
Abstract
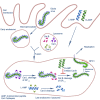
The 2014-2016 West Africa Ebola virus (EBOV) outbreak coupled with the most recent outbreaks in Central Africa underscore the need to develop effective treatment strategies against EBOV. Although several therapeutic options have shown great potential, developing a wider breadth of countermeasures would increase our efforts to combat the highly lethal EBOV. Here we show that human cathelicidin antimicrobial peptide (AMP) LL-37 and engineered LL-37 AMPs inhibit the infection of recombinant virus pseudotyped with EBOV glycoprotein (GP) and the wild-type EBOV. These AMPs target EBOV infection at the endosomal cell-entry step by impairing cathepsin B-mediated processing of EBOV GP. Furthermore, two engineered AMPs containing D-amino acids are particularly potent in blocking EBOV infection in comparison with other AMPs, most likely owing to their resistance to intracellular enzymatic degradation. Our results identify AMPs as a novel class of anti-EBOV therapeutics and demonstrate the feasibility of engineering AMPs for improved therapeutic efficacy.
Keywords: Drugs; Molecular Biology; Viral Microbiology.
© 2020 The Authors.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Agerberth B., Charo J., Werr J., Olsson B., Idali F., Lindbom L., Kiessling R., Jornvall H., Wigzell H., Gudmundsson G.H. The human antimicrobial and chemotactic peptides LL-37 and alpha-defensins are expressed by specific lymphocyte and monocyte populations. Blood. 2000;96:3086–3093. - PubMed
-
- Andrault P.M., Samsonov S.A., Weber G., Coquet L., Nazmi K., Bolscher J.G., Lalmanach A.C., Jouenne T., Bromme D., Pisabarro M.T. Antimicrobial peptide LL-37 is both a substrate of cathepsins S and K and a selective inhibitor of cathepsin L. Biochemistry. 2015;54:2785–2798. - PubMed
-
- Bergman P., Walter-Jallow L., Broliden K., Agerberth B., Soderlund J. The antimicrobial peptide LL-37 inhibits HIV-1 replication. Curr. HIV Res. 2007;5:410–415. - PubMed
-
- Bixler S.L., Bocan T.M., Wells J., Wetzel K.S., Van Tongeren S.A., Dong L., Garza N.L., Donnelly G., Cazares L.H., Nuss J. Efficacy of favipiravir (T-705) in nonhuman primates infected with Ebola virus or Marburg virus. Antivir. Res. 2018;151:97–104. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

