Direct and Intervertebral Disc-Mediated Sensitization of Dorsal Root Ganglion Neurons by Hypoxia and Low pH
- PMID: 32252154
- PMCID: PMC7136118
- DOI: 10.14245/ns.2040052.026
Direct and Intervertebral Disc-Mediated Sensitization of Dorsal Root Ganglion Neurons by Hypoxia and Low pH
Abstract
Objective: Ischemia-related risk factors are consistently correlated with discogenic pain, but it remains unclear how the ischemia-associated hypoxia and acidosis influence the peripheral sensory nervous system, namely the dorsal root ganglion (DRG), either directly or indirectly via intervertebral disc (IVD) mediation.
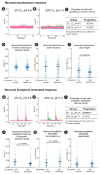
Methods: Bovine tail IVD organ cultures were preconditioned in different hypoxic and/or acidic conditions for 3 days to collect the conditioned medium (CM). The DRG-derived ND7/23 cells were either treated by the IVD CM or directly stimulated by hypoxic and/or acidic conditions. Neuronal sensitization was evaluated using calcium imaging (Fluo-4) after 3 days.
Results: We found that direct exposure of DRG cell line to hypoxia and acidosis increased both spontaneous and bradykinin-stimulated calcium response compared to normoxia-neutral pH cultures. Hypoxia and low pH in combination showed stronger effect than either parameter on its own. Indirect exposure of DRG to hypoxia-acidosis-stressed IVD CM also increased spontaneous and bradykinin-stimulated response, but to a lower extent than direct exposure. The impact of direct hypoxia and acidosis on DRG was validated in a primary sheep DRG cell culture, showing the same trend.
Conclusion: Our data suggest that targeting hypoxia and acidosis stresses both in IVD and DRG could be a relevant objective in discogenic pain treatment.
Keywords: Acidosis; Calcium Imaging; Dorsal root ganglion; Hypoxia; Intervertebral disc; Low back pain.
Conflict of interest statement
The authors have nothing to disclose.
Figures










Similar articles
-
Hypoxic stress enhances extension and branching of dorsal root ganglion neuronal outgrowth.JOR Spine. 2020 May 4;3(2):e1090. doi: 10.1002/jsp2.1090. eCollection 2020 Jun. JOR Spine. 2020. PMID: 32613165 Free PMC article.
-
Degenerative IVD conditioned media and acidic pH sensitize sensory neurons to cyclic tensile strain.J Orthop Res. 2021 Jun;39(6):1192-1203. doi: 10.1002/jor.24682. Epub 2020 May 4. J Orthop Res. 2021. PMID: 32255531 Free PMC article.
-
The influence of intervertebral disc overloading on nociceptor calcium flickering.JOR Spine. 2023 Jun 9;6(3):e1267. doi: 10.1002/jsp2.1267. eCollection 2023 Sep. JOR Spine. 2023. PMID: 37780827 Free PMC article.
-
Distribution and immunocytochemical characterization of dorsal root ganglion neurons innervating the lumbar intervertebral disc in rats: a review.Life Sci. 2004 Apr 9;74(21):2627-42. doi: 10.1016/j.lfs.2004.01.008. Life Sci. 2004. PMID: 15041445 Review.
-
Stem Cell Therapies for Treatment of Discogenic Low Back Pain: a Comprehensive Review.Curr Pain Headache Rep. 2019 Jul 29;23(9):65. doi: 10.1007/s11916-019-0804-y. Curr Pain Headache Rep. 2019. PMID: 31359164 Review.
Cited by
-
Celecoxib alleviates nociceptor sensitization mediated by interleukin-1beta-primed annulus fibrosus cells.Eur Spine J. 2023 Jun;32(6):2048-2058. doi: 10.1007/s00586-023-07672-x. Epub 2023 Apr 18. Eur Spine J. 2023. PMID: 37071156
-
Narrative Review of Pathophysiology and Endoscopic Management of Basivertebral and Sinuvertebral Neuropathy for Chronic Back Pain.J Korean Neurosurg Soc. 2023 Jul;66(4):344-355. doi: 10.3340/jkns.2022.0140. Epub 2022 Nov 28. J Korean Neurosurg Soc. 2023. PMID: 36444421 Free PMC article.
-
Dynamic Immune Landscape and VZV-Specific T Cell Responses in Patients With Herpes Zoster and Postherpetic Neuralgia.Front Immunol. 2022 Jun 1;13:887892. doi: 10.3389/fimmu.2022.887892. eCollection 2022. Front Immunol. 2022. PMID: 35720399 Free PMC article.
-
In vivo measurement of intradiscal pressure changes related to thrust and non-thrust spinal manipulation in an animal model: a pilot study.Chiropr Man Therap. 2022 Sep 6;30(1):36. doi: 10.1186/s12998-022-00445-1. Chiropr Man Therap. 2022. PMID: 36068588 Free PMC article.
-
Current Knowledge and Future Therapeutic Prospects in Symptomatic Intervertebral Disc Degeneration.Yonsei Med J. 2022 Mar;63(3):199-210. doi: 10.3349/ymj.2022.63.3.199. Yonsei Med J. 2022. PMID: 35184422 Free PMC article. Review.
References
-
- GBD 2016 Disease and Injury Incidence and Prevalence Collaborators Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390:1211–59. - PMC - PubMed
-
- DePalma MJ, Ketchum JM, Saullo T. What is the source of chronic low back pain and does age play a role? Pain Med. 2011;12:224–33. - PubMed
-
- Hangai M, Kaneoka K, Kuno S, et al. Factors associated with lumbar intervertebral disc degeneration in the elderly. Spine J. 2008;8:732–40. - PubMed
-
- Kauppila LI. Atherosclerosis and disc degeneration/low-back pain--a systematic review. Eur J Vasc Endovasc Surg. 2009;37:661–70. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

