A Mini-Review on Anion Exchange and Chelating Polymers for Applications in Hydrometallurgy, Environmental Protection, and Biomedicine
- PMID: 32252240
- PMCID: PMC7240740
- DOI: 10.3390/polym12040784
A Mini-Review on Anion Exchange and Chelating Polymers for Applications in Hydrometallurgy, Environmental Protection, and Biomedicine
Abstract
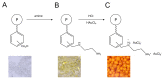
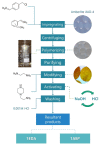
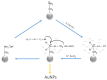
The rapidly increasing demand for technologies aiming to resolve challenges of separations and environmental protection causes a sharp increase in the demand for ion exchange (IX) and chelating polymers. These unique materials can offer target-selective adsorption properties vital for the removal or recovery of harmful and precious materials, where trace concentrations thereof make other techniques insufficient. Hence, recent achievements in syntheses of IX and chelating resins designed and developed in our research group are discussed within this mini-review. The aim of the present work is to reveal that, due to the diversified and unique physiochemical characteristics of the proposed materials, they are not limited to traditional separation techniques and could be used in multifunctional areas of applications, including catalysis, heat management, and biomedicine.
Keywords: cold atmospheric pressure plasma; polymeric nanocomposite; precious metals; resins.
Conflict of interest statement
The Authors declare no conflict of interest.
Figures
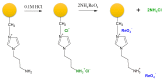



Similar articles
-
[Magnetic ion imprinting techniques for the separation and analysis of elemental speciation].Se Pu. 2022 Nov;40(11):979-987. doi: 10.3724/SP.J.1123.2022.07013. Se Pu. 2022. PMID: 36351806 Free PMC article. Review. Chinese.
-
Characterization and Optimization of Polymeric Bispicolamine Chelating Resin: Performance Evaluation via RSM Using Copper in Acid Liquors as a Model Substrate through Ion Exchange Method.Molecules. 2022 Oct 25;27(21):7210. doi: 10.3390/molecules27217210. Molecules. 2022. PMID: 36364043 Free PMC article.
-
Phenol removal from aqueous solution by adsorption and ion exchange mechanisms onto polymeric resins.J Colloid Interface Sci. 2009 Oct 15;338(2):402-9. doi: 10.1016/j.jcis.2009.06.062. Epub 2009 Jul 3. J Colloid Interface Sci. 2009. PMID: 19679317
-
Polymer-Supported Phosphoric, Phosphonic and Phosphinic Acids-From Synthesis to Properties and Applications in Separation Processes.Molecules. 2020 Sep 15;25(18):4236. doi: 10.3390/molecules25184236. Molecules. 2020. PMID: 32942756 Free PMC article. Review.
-
Polymeric Materials for Rare Earth Elements Recovery.Gels. 2023 Sep 24;9(10):775. doi: 10.3390/gels9100775. Gels. 2023. PMID: 37888349 Free PMC article. Review.
Cited by
-
Application of Amberlite IRA 402 Resin Adsorption and Laccase Treatment for Acid Blue 113 Removal from Aqueous Media.Polymers (Basel). 2021 Nov 18;13(22):3991. doi: 10.3390/polym13223991. Polymers (Basel). 2021. PMID: 34833290 Free PMC article.
-
Adsorption of Toxic Metals Using Hydrous Ferric Oxide Nanoparticles Embedded in Hybrid Ion-Exchange Resins.Materials (Basel). 2024 Mar 1;17(5):1168. doi: 10.3390/ma17051168. Materials (Basel). 2024. PMID: 38473639 Free PMC article.
-
Synthesis and Characterization of Chelating Hyperbranched Polyester Nanoparticles for Cd(II) Ion Removal from Water.Molecules. 2022 Jun 7;27(12):3656. doi: 10.3390/molecules27123656. Molecules. 2022. PMID: 35744784 Free PMC article.
References
-
- Syed S. Recovery of gold from secondary sources—A review. Hydrometallurgy. 2012;115:30–51. doi: 10.1016/j.hydromet.2011.12.012. - DOI
-
- Liang P., Yuana L., Yangb X., Zhouc S., Huang X. Coupling ion-exchangers with inexpensive activated carbon fiber electrodes to enhance the performance of capacitive deionization cells for domestic wastewater desalination. Water Res. 2013;47:2523–2530. doi: 10.1016/j.watres.2013.02.037. - DOI - PubMed
-
- Harris N.D. Process for Preparing Chloromethylated Polystyrene-Divinylbenzene Copolymer. 3872067. U.S. Patent. 1975 Mar 18;
-
- Parajuli D., Kunathai K., Adhikari C.R., Inoue K., Ohto K., Kawakita H., Funaoka M., Hirota K. Total recovery of gold, palladium, and platinum using lignophenol derivative. Miner. Eng. 2009;22:1173–1178. doi: 10.1016/j.mineng.2009.06.003. - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

