Glycomics Microarrays Reveal Differential In Situ Presentation of the Biofilm Polysaccharide Poly- N-acetylglucosamine on Acinetobacter baumannii and Staphylococcus aureus Cell Surfaces
- PMID: 32252300
- PMCID: PMC7177611
- DOI: 10.3390/ijms21072465
Glycomics Microarrays Reveal Differential In Situ Presentation of the Biofilm Polysaccharide Poly- N-acetylglucosamine on Acinetobacter baumannii and Staphylococcus aureus Cell Surfaces
Abstract
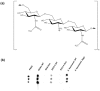
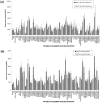
The biofilm component poly-N-acetylglucosamine (PNAG) is an important virulence determinant in medical-device-related infections caused by ESKAPE group pathogens including Gram-positive Staphylococcus aureus and Gram-negative Acinetobacter baumannii. PNAG presentation on bacterial cell surfaces and its accessibility for host interactions are not fully understood. We employed a lectin microarray to examine PNAG surface presentation and interactions on methicillin-sensitive (MSSA) and methicillin-resistant S. aureus (MRSA) and a clinical A. baumannii isolate. Purified PNAG bound to wheatgerm agglutinin (WGA) and succinylated WGA (sWGA) lectins only. PNAG was the main accessible surface component on MSSA but was relatively inaccessible on the A. baumannii surface, where it modulated the presentation of other surface molecules. Carbohydrate microarrays demonstrated similar specificities of S. aureus and A. baumannii for their most intensely binding carbohydrates, including 3' and 6'sialyllactose, but differences in moderately binding ligands, including blood groups A and B. An N-acetylglucosamine-binding lectin function which binds to PNAG identified on the A. baumannii cell surface may contribute to biofilm structure and PNAG surface presentation on A. baumannii. Overall, these data indicated differences in PNAG presentation and accessibility for interactions on Gram-positive and Gram-negative cell surfaces which may play an important role in biofilm-mediated pathogenesis.
Keywords: Acinetobacter baumannii; PNAG; Staphylococcus aureus; bacterial adhesins; biofilm; glycomics microarrays; lectin; poly-N-acetylglucosamine; polysaccharide.
Conflict of interest statement
Gerald B. Pier is an inventor of intellectual properties (human monoclonal antibody to PNAG and PNAG vaccines) that are licensed by Brigham and Women’s Hospital to Alopexx Vaccine, LLC, and Alopexx Pharmaceuticals, LLC, entities in which Gerald B. Pier also holds equity. As an inventor of intellectual properties, Gerald B. Pier also has the right to receive a share of licensing-related income (royalties, fees) through Brigham and Women’s Hospital from Alopexx Pharmaceuticals, LLC, and Alopexx Vaccine, LLC. Gerald B. Pier’s interests were reviewed and are managed by the Brigham and Women’s Hospital and Partners Healthcare in accordance with their conflict of interest policies. The other authors declare no competing financial interests. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures




References
-
- World Health Organisation . Report on the Burden of Endemic Health Care-Associated Infection Worldwide. World Health Organisation; Geneva, Switzerland: 2011.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

