Modulation of STAT3 Signaling, Cell Redox Defenses and Cell Cycle Checkpoints by β-Caryophyllene in Cholangiocarcinoma Cells: Possible Mechanisms Accounting for Doxorubicin Chemosensitization and Chemoprevention
- PMID: 32252311
- PMCID: PMC7226839
- DOI: 10.3390/cells9040858
Modulation of STAT3 Signaling, Cell Redox Defenses and Cell Cycle Checkpoints by β-Caryophyllene in Cholangiocarcinoma Cells: Possible Mechanisms Accounting for Doxorubicin Chemosensitization and Chemoprevention
Abstract
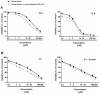
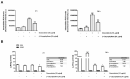
Cholangiocarcinoma (CCA) is an aggressive group of biliary tract cancers, characterized by late diagnosis, low effective chemotherapies, multidrug resistance, and poor outcomes. In the attempt to identify new therapeutic strategies for CCA, we studied the antiproliferative activity of a combination between doxorubicin and the natural sesquiterpene β-caryophyllene in cholangiocarcinoma Mz-ChA-1 cells and nonmalignant H69 cholangiocytes, under both long-term and metronomic schedules. The modulation of STAT3 signaling, oxidative stress, DNA damage response, cell cycle progression and apoptosis was investigated as possible mechanisms of action. β-caryophyllene was able to synergize the cytotoxicity of low dose doxorubicin in Mz-ChA-1 cells, while producing cytoprotective effects in H69 cholangiocytes, mainly after a long-term exposure of 24 h. The mechanistic analysis highlighted that the sesquiterpene induced a cell cycle arrest in G2/M phase along with the doxorubicin-induced accumulation in S phase, reduced the γH2AX and GSH levels without affecting GSSG. ROS amount was partly lowered by the combination in Mz-ChA-1 cells, while increased in H69 cells. A lowered expression of doxorubicin-induced STAT3 activation was found in the presence of β-caryophyllene in both cancer and normal cholangiocytes. These networking effects resulted in an increased apoptosis rate in Mz-ChA-1 cells, despite a lowering in H69 cholangiocytes. This evidence highlighted a possible role of STAT3 as a final effector of a complex network regulated by β-caryophyllene, which leads to an enhanced doxorubicin-sensitivity of cholangiocarcinoma cells and a lowered chemotherapy toxicity in nonmalignant cholangiocytes, thus strengthening the interest for this natural sesquiterpene as a dual-acting chemosensitizing and chemopreventive agent.
Keywords: GSH depletion; H2AX phosphorylation; STAT3 signaling; apoptosis; caryophyllane sesquiterpenes; cell cycle checkpoint; chemoprevention; cholangiocytes; genoprotective effects; liver cancer; metronomic schedule.
Conflict of interest statement
The authors declare no conflict of interest.
Figures












Similar articles
-
Chemopreventive Potential of Caryophyllane Sesquiterpenes: An Overview of Preliminary Evidence.Cancers (Basel). 2020 Oct 18;12(10):3034. doi: 10.3390/cancers12103034. Cancers (Basel). 2020. PMID: 33081075 Free PMC article. Review.
-
Potentiation of Low-Dose Doxorubicin Cytotoxicity by Affecting P-Glycoprotein through Caryophyllane Sesquiterpenes in HepG2 Cells: an in Vitro and in Silico Study.Int J Mol Sci. 2020 Jan 17;21(2):633. doi: 10.3390/ijms21020633. Int J Mol Sci. 2020. PMID: 31963614 Free PMC article.
-
Cryptotanshinone induces cell cycle arrest and apoptosis through the JAK2/STAT3 and PI3K/Akt/NFκB pathways in cholangiocarcinoma cells.Drug Des Devel Ther. 2017 Jun 15;11:1753-1766. doi: 10.2147/DDDT.S132488. eCollection 2017. Drug Des Devel Ther. 2017. PMID: 28670110 Free PMC article.
-
Sorafenib Chemosensitization by Caryophyllane Sesquiterpenes in Liver, Biliary, and Pancreatic Cancer Cells: The Role of STAT3/ABC Transporter Axis.Pharmaceutics. 2022 Jun 14;14(6):1264. doi: 10.3390/pharmaceutics14061264. Pharmaceutics. 2022. PMID: 35745837 Free PMC article.
-
Natural Sesquiterpene Lactones Enhance Chemosensitivity of Tumor Cells through Redox Regulation of STAT3 Signaling.Oxid Med Cell Longev. 2019 Oct 28;2019:4568964. doi: 10.1155/2019/4568964. eCollection 2019. Oxid Med Cell Longev. 2019. PMID: 31781335 Free PMC article. Review.
Cited by
-
Chemopreventive Potential of Caryophyllane Sesquiterpenes: An Overview of Preliminary Evidence.Cancers (Basel). 2020 Oct 18;12(10):3034. doi: 10.3390/cancers12103034. Cancers (Basel). 2020. PMID: 33081075 Free PMC article. Review.
-
Pinus mugo Essential Oil Impairs STAT3 Activation through Oxidative Stress and Induces Apoptosis in Prostate Cancer Cells.Molecules. 2022 Jul 28;27(15):4834. doi: 10.3390/molecules27154834. Molecules. 2022. PMID: 35956786 Free PMC article.
-
The Potential Therapeutic Role of Beta-Caryophyllene as a Chemosensitizer and an Inhibitor of Angiogenesis in Cancer.Molecules. 2025 Apr 14;30(8):1751. doi: 10.3390/molecules30081751. Molecules. 2025. PMID: 40333803 Free PMC article. Review.
-
Biochemical and functional properties of vesicles from planktonic and biofilm phenotypes of Limosilactobacillus reuteri DSM 17938.Sci Rep. 2025 May 29;15(1):18889. doi: 10.1038/s41598-025-03823-w. Sci Rep. 2025. PMID: 40442239 Free PMC article.
-
Characterization of the Chemopreventive Properties of Cannabis sativa L. Inflorescences from Monoecious Cultivars Grown in Central Italy.Plants (Basel). 2023 Nov 9;12(22):3814. doi: 10.3390/plants12223814. Plants (Basel). 2023. PMID: 38005711 Free PMC article.
References
-
- Fava G., DeMorrow S., Gaudio E., Franchitto A., Onori P., Carpino G., Glaser S., Francis H., Coufal M., Marucci L., et al. Endothelin inhibits cholangiocarcinoma growth by a decrease in the vascular endothelial growth factor expression. Liver Int. 2009;29:1031–1042. doi: 10.1111/j.1478-3231.2009.01997.x. - DOI - PMC - PubMed
-
- Lozano E., Macias R.I., Monte M.J., Asensio M., Del Carmen S., Sanchez-Vicente L., Alonso-Peña M., Al-Abdulla R., Munoz-Garrido P., Satriano L., et al. Causes of hOCT1-Dependent Cholangiocarcinoma Resistance to Sorafenib and Sensitization by Tumor-Selective Gene Therapy. Hepatology. 2019;70:1246–1261. doi: 10.1002/hep.30656. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

