New Insights from IGF-IR Stimulating Activity Analyses: Pathological Considerations
- PMID: 32252327
- PMCID: PMC7226833
- DOI: 10.3390/cells9040862
New Insights from IGF-IR Stimulating Activity Analyses: Pathological Considerations
Abstract
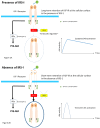
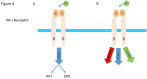
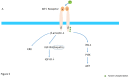
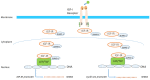
Insulin-like growth factor-I (IGF-I) and insulin-like growth factor-II (IGF-II) play a crucial factor in the growth, differentiation and survival of cells in health and disease. IGF-I and IGF-II primarily activate the IGF-I receptor (IGF-IR), which is present on the cell surface. Activation of the IGF-IR stimulates multiple pathways which finally results in multiple biological effects in a variety of tissues and cells. In addition, activation of the IGF-IR has been found to be essential for the growth of cancers. The conventional view in the past was that the IGF-IR was exclusively a tyrosine kinase receptor and that phosphorylation of tyrosine residues, after binding of IGF-I to the IGF-IR, started a cascade of post-receptor events. Recent research has shown that this view was too simplistic. It has been found that the IGF-IR also has kinase-independent functions and may even emit signals in the unoccupied state through some yet-to-be-defined non-canonical pathways. The IGF-IR may further form hybrids with the insulin receptors but also with receptor tyrosine kinases (RTKs) outside the insulin-IGF system. In addition, the IGF-IR has extensive cross-talk with many other receptor tyrosine kinases and their downstream effectors. Moreover, there is now emerging evidence that the IGF-IR utilizes parts of the G-protein coupled receptor (GPCR) pathways: the IGF-IR can be considered as a functional RTK/GPCR hybrid, which integrates the kinase signaling with some IGF-IR mediated canonical GPCR characteristics. Like the classical GPCRs the IGF-IR can also show homologous and heterologous desensitization. Recently, it has been found that after activation by a ligand, the IGF-IR may be translocated into the nucleus and function as a transcriptional cofactor. Thus, in recent years, it has become clear that the IGF-IR signaling pathways are much more complex than first thought. Therefore a big challenge for the (near) future will be how all the new knowledge about IGF-IR signaling can be translated into the clinical practice and improve diagnosis and treatment of diseases.
Keywords: G-proteins; GPCRs; IGF-I; IGF-II; IGF-IR; IRs; functional RTK/GPCR hybrid; hybrids; insulin; nuclear translocation; phosphorylation; tyrosine kinase receptor; β-arrestins.
Conflict of interest statement
The author declares no conflict of interest.
Figures



References
-
- Jones J.I., Clemmons D.R. Insulin-like growth factors and their binding proteins: Biological actions. Endocr. Rev. 1995;16:3–34. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

