PET Imaging of the Adenosine A2A Receptor in the Rotenone-Based Mouse Model of Parkinson's Disease with [18F]FESCH Synthesized by a Simplified Two-Step One-Pot Radiolabeling Strategy
- PMID: 32252340
- PMCID: PMC7180622
- DOI: 10.3390/molecules25071633
PET Imaging of the Adenosine A2A Receptor in the Rotenone-Based Mouse Model of Parkinson's Disease with [18F]FESCH Synthesized by a Simplified Two-Step One-Pot Radiolabeling Strategy
Abstract
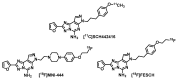
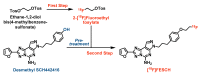
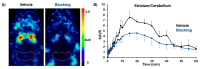
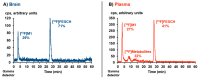
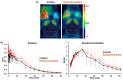
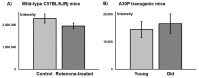
The adenosine A2A receptor (A2AR) is regarded as a particularly appropriate target for non-dopaminergic treatment of Parkinson's disease (PD). An increased A2AR availability has been found in the human striatum at early stages of PD and in patients with PD and dyskinesias. The aim of this small animal positron emission tomography/magnetic resonance (PET/MR) imaging study was to investigate whether rotenone-treated mice reflect the aspect of striatal A2AR upregulation in PD. For that purpose, we selected the known A2AR-specific radiotracer [18F]FESCH and developed a simplified two-step one-pot radiosynthesis. PET images showed a high uptake of [18F]FESCH in the mouse striatum. Concomitantly, metabolism studies with [18F]FESCH revealed the presence of a brain-penetrant radiometabolite. In rotenone-treated mice, a slightly higher striatal A2AR binding of [18F]FESCH was found. Nonetheless, the correlation between the increased A2AR levels within the proposed PD animal model remains to be further investigated.
Keywords: PET imaging; Parkinson’s disease; [18F]FESCH; adenosine A2A receptor; rotenone-based mouse model; two-step one-pot radiosynthesis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Gerlach M., Reichmann H., Riederer P. Die Parkinson-Krankheit: Grundlagen, Klinik, Therapie. Springer; Wien, Austria: New York, NY, USA: 2007. p. 453.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

