Metabolic Heterogeneity of Cancer Cells: An Interplay between HIF-1, GLUTs, and AMPK
- PMID: 32252351
- PMCID: PMC7226606
- DOI: 10.3390/cancers12040862
Metabolic Heterogeneity of Cancer Cells: An Interplay between HIF-1, GLUTs, and AMPK
Abstract
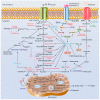
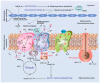
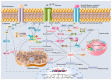
It has been long recognized that cancer cells reprogram their metabolism under hypoxia conditions due to a shift from oxidative phosphorylation (OXPHOS) to glycolysis in order to meet elevated requirements in energy and nutrients for proliferation, migration, and survival. However, data accumulated over recent years has increasingly provided evidence that cancer cells can revert from glycolysis to OXPHOS and maintain both reprogrammed and oxidative metabolism, even in the same tumor. This phenomenon, denoted as cancer cell metabolic plasticity or hybrid metabolism, depends on a tumor micro-environment that is highly heterogeneous and influenced by an intensity of vasculature and blood flow, oxygen concentration, and nutrient and energy supply, and requires regulatory interplay between multiple oncogenes, transcription factors, growth factors, and reactive oxygen species (ROS), among others. Hypoxia-inducible factor-1 (HIF-1) and AMP-activated protein kinase (AMPK) represent key modulators of a switch between reprogrammed and oxidative metabolism. The present review focuses on cross-talks between HIF-1, glucose transporters (GLUTs), and AMPK with other regulatory proteins including oncogenes such as c-Myc, p53, and KRAS; growth factor-initiated protein kinase B (PKB)/Akt, phosphatydyl-3-kinase (PI3K), and mTOR signaling pathways; and tumor suppressors such as liver kinase B1 (LKB1) and TSC1 in controlling cancer cell metabolism. The multiple switches between metabolic pathways can underlie chemo-resistance to conventional anti-cancer therapy and should be taken into account in choosing molecular targets to discover novel anti-cancer drugs.
Keywords: AMPK; GLUTs; HIF-1; OXPHOS; cancer metabolism.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Modeling the Genetic Regulation of Cancer Metabolism: Interplay between Glycolysis and Oxidative Phosphorylation.Cancer Res. 2017 Apr 1;77(7):1564-1574. doi: 10.1158/0008-5472.CAN-16-2074. Epub 2017 Feb 15. Cancer Res. 2017. PMID: 28202516 Free PMC article.
-
Metabolic evolutionary roots of the macrophage immune response in amoeba-bacteria interactions: The conserved role of hypoxia-induced Factor and AMP kinase.Acta Biochim Pol. 2021 Aug 10;68(3):457-476. doi: 10.18388/abp.2020_5683. Acta Biochim Pol. 2021. PMID: 34374500 Review.
-
Lactic Acidosis in the Presence of Glucose Diminishes Warburg Effect in Lung Adenocarcinoma Cells.Front Oncol. 2020 Jun 12;10:807. doi: 10.3389/fonc.2020.00807. eCollection 2020. Front Oncol. 2020. PMID: 32596143 Free PMC article.
-
p53 and glucose metabolism: an orchestra to be directed in cancer therapy.Pharmacol Res. 2018 May;131:75-86. doi: 10.1016/j.phrs.2018.03.015. Epub 2018 Mar 23. Pharmacol Res. 2018. PMID: 29580896 Review.
-
Recent advances in the role of AMP-activated protein kinase in metabolic reprogramming of metastatic cancer cells: targeting cellular bioenergetics and biosynthetic pathways for anti-tumor treatment.J Physiol Pharmacol. 2018 Jun;69(3). doi: 10.26402/jpp.2018.3.07. Epub 2018 Sep 28. J Physiol Pharmacol. 2018. PMID: 30279304 Review.
Cited by
-
TRPM7 silencing modulates glucose metabolic reprogramming to inhibit the growth of ovarian cancer by enhancing AMPK activation to promote HIF-1α degradation.J Exp Clin Cancer Res. 2022 Jan 31;41(1):44. doi: 10.1186/s13046-022-02252-1. J Exp Clin Cancer Res. 2022. PMID: 35101076 Free PMC article.
-
New Immunometabolic Strategy Based on Cell Type-Specific Metabolic Reprogramming in the Tumor Immune Microenvironment.Cells. 2022 Feb 22;11(5):768. doi: 10.3390/cells11050768. Cells. 2022. PMID: 35269390 Free PMC article. Review.
-
The Metabolic Landscape of Cancer Stem Cells: Insights and Implications for Therapy.Cells. 2025 May 15;14(10):717. doi: 10.3390/cells14100717. Cells. 2025. PMID: 40422220 Free PMC article. Review.
-
Role of Mitochondria in Radiation Responses: Epigenetic, Metabolic, and Signaling Impacts.Int J Mol Sci. 2021 Oct 13;22(20):11047. doi: 10.3390/ijms222011047. Int J Mol Sci. 2021. PMID: 34681703 Free PMC article. Review.
-
Regulation of Hypoxia Dependent Reprogramming of Cancer Metabolism: Role of HIF-1 and Its Potential Therapeutic Implications in Leukemia.Asian Pac J Cancer Prev. 2024 Apr 1;25(4):1121-1134. doi: 10.31557/APJCP.2024.25.4.1121. Asian Pac J Cancer Prev. 2024. PMID: 38679971 Free PMC article. Review.
References
-
- Ralph S.J., Rodriguez-Enriguez S., Neuzil J., Saavedra E., Moreno-Sanchez R. The causes of cancer revisited: “Mitochondrial malignancy” and ROS-induced oncogenic transformation—Why mitochondria are targets for cancer therapy. Mol. Asp. Med. 2010;31:145–170. doi: 10.1016/j.mam.2010.02.008. - DOI - PubMed
-
- Vaupel P., Multhoff G. Hypoxia-/HIF-1α-driven factors of the tumor microenvironment impeding antitumor immune response and promoting malignant progression. Adv. Exp. Med. Biol. 2018;1072:171–175. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

