Overexpression of MADS-box Gene AGAMOUS-LIKE 12 Activates Root Development in Juglans sp. and Arabidopsis thaliana
- PMID: 32252382
- PMCID: PMC7238194
- DOI: 10.3390/plants9040444
Overexpression of MADS-box Gene AGAMOUS-LIKE 12 Activates Root Development in Juglans sp. and Arabidopsis thaliana
Abstract
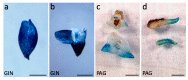
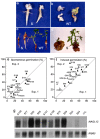
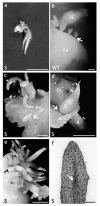
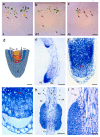
Until recently, the roles of plant MADS-box genes have mainly been characterized during inflorescence and flower differentiation. In order to precise the roles of AGAMOUS-LIKE 12, one of the few MADS-box genes preferentially expressed in roots, we placed its cDNA under the control of the double 35S CaMV promoter to produce transgenic walnut tree and Arabidopsis plants. In Juglans sp., transgenic somatic embryos showed significantly higher germination rates but abnormal development of their shoot apex prevented their conversion into plants. In addition, a wide range of developmental abnormalities corresponding to ectopic root-like structures affected the transgenic lines suggesting partial reorientations of the embryonic program toward root differentiation. In Arabidopsis, AtAGL12 overexpression lead to the production of faster growing plants presenting dramatically wider and shorter root phenotypes linked to increased meristematic cell numbers within the root apex. In the upper part of the roots, abnormal cell divisions patterns within the pericycle layer generated large ectopic cell masses that did not prevent plants to grow. Taken together, our results confirm in both species that AGL12 positively regulates root meristem cell division and promotes overall root vascular tissue formation. Genetic engineering of AGL12 expression levels could be useful to modulate root architecture and development.
Keywords: cell differentiation; cell division; root meristem; transcription factor; transgenic plant.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Alvarez-Buylla E.R., Liljegren S.J., Pelaz S., Gold S.E., Burgeff C., Ditta G.S., Vergara-Silva F., Yanofsky M.F. MADS-box gene evolution beyond flowers: Expression in pollen, endosperm, guard cells, roots and trichomes. Plant J. 2000;24:457–466. doi: 10.1046/j.1365-313x.2000.00891.x. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

