Diagnostic and Therapeutic Potential of TSPO Studies Regarding Neurodegenerative Diseases, Psychiatric Disorders, Alcohol Use Disorders, Traumatic Brain Injury, and Stroke: An Update
- PMID: 32252470
- PMCID: PMC7226777
- DOI: 10.3390/cells9040870
Diagnostic and Therapeutic Potential of TSPO Studies Regarding Neurodegenerative Diseases, Psychiatric Disorders, Alcohol Use Disorders, Traumatic Brain Injury, and Stroke: An Update
Abstract
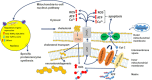
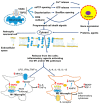
Neuroinflammation and cell death are among the common symptoms of many central nervous system diseases and injuries. Neuroinflammation and programmed cell death of the various cell types in the brain appear to be part of these disorders, and characteristic for each cell type, including neurons and glia cells. Concerning the effects of 18-kDa translocator protein (TSPO) on glial activation, as well as being associated with neuronal cell death, as a response mechanism to oxidative stress, the changes of its expression assayed with the aid of TSPO-specific positron emission tomography (PET) tracers' uptake could also offer evidence for following the pathogenesis of these disorders. This could potentially increase the number of diagnostic tests to accurately establish the stadium and development of the disease in question. Nonetheless, the differences in results regarding TSPO PET signals of first and second generations of tracers measured in patients with neurological disorders versus healthy controls indicate that we still have to understand more regarding TSPO characteristics. Expanding on investigations regarding the neuroprotective and healing effects of TSPO ligands could also contribute to a better understanding of the therapeutic potential of TSPO activity for brain damage due to brain injury and disease. Studies so far have directed attention to the effects on neurons and glia, and processes, such as death, inflammation, and regeneration. It is definitely worthwhile to drive such studies forward. From recent research it also appears that TSPO ligands, such as PK11195, Etifoxine, Emapunil, and 2-Cl-MGV-1, demonstrate the potential of targeting TSPO for treatments of brain diseases and disorders.
Keywords: PET tracing; TSPO; astrocytes; brain disease; brain disorders; cell death; drug development; microglia; microglia activation; neurons; regeneration.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Gavish M., Bachman I., Shoukrun R., Katz Y., Veenman L., Weisinger G., Weizman A. Enigma of the peripheral benzodiazepine receptor. Pharmacol. Rev. 1999;51:629–650. - PubMed
-
- Dimitrova-Shumkovska J., Veenman L., Ristoski T., Leschiner S., Gavish M. Dimethylbenz[alpha]anthracene induces oxidative stress and reduces the binding capacity of the mitochondrial 18-kDa translocator protein in rat aorta. Drug Chem. Toxicol. 2010;33:337–347. doi: 10.3109/01480540903483441. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

