Impact of Type 2 Diabetes Mellitus on Human Bone Marrow Stromal Cell Number and Phenotypic Characteristics
- PMID: 32252490
- PMCID: PMC7177361
- DOI: 10.3390/ijms21072476
Impact of Type 2 Diabetes Mellitus on Human Bone Marrow Stromal Cell Number and Phenotypic Characteristics
Abstract
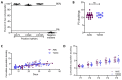
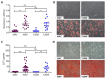
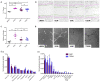
Human bone marrow-derived mesenchymal stromal cells (MSCs) have been investigated in numerous disease settings involving impaired regeneration because of the crucial role they play in tissue maintenance and repair. Considering the number of comorbidities associated with type 2 diabetes mellitus (T2DM), the hypothesis that MSCs mediate these comorbidities via a reduction in their native maintenance and repair activities is an intriguing line of inquiry. Here, it is demonstrated that the number of bone marrow-derived MSCs in people with T2DM was reduced compared to that of age-matched control (AMC) donors and that this was due to a specific decrease in the number of MSCs with osteogenic capacity. There were no differences in MSC cell surface phenotype or in MSC expansion, differentiation, or angiogenic or migratory capacity from donors living with T2DM as compared to AMCs. These findings elucidate the basic biology of MSCs and their potential as mediators of diabetic comorbidities, especially osteopathies, and provide insight into donor choice for MSC-based clinical trials. This study suggests that any role of bone marrow MSCs as a mediator of T2DM comorbidity is likely due to a reduction in the osteoprogenitor population size and not due to a permanent alteration to the MSCs' capacity to maintain tissue homeostasis through expansion and differentiation.
Keywords: adult stem cells; bone marrow stromal cells; mesenchymal stem cells; mesenchymal stromal cells; type 2 diabetes mellitus.
Conflict of interest statement
The authors declare no conflict of interest to disclose with the exception of Prof. Timothy O’Brien who is a founder, director, and equity holder in Orbsen Therapeutics. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures


References
-
- Dominici M., Le Blanc K., Mueller I., Slaper-Cortenbach I., Marini F., Krause D.S., Deans R.J., Keating A., Prockop D.J., Horwitz E.M. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. - DOI - PubMed
-
- Consentius C., Mirenska A., Jurisch A., Reinke S., Scharm M., Zenclussen A.C., Hennig C., Volk H.-D. In situ detection of CD73+ CD90+ CD105+ lineage: Mesenchymal stromal cells in human placenta and bone marrow specimens by chipcytometry. Cytom. Part A. 2018;93:889–893. doi: 10.1002/cyto.a.23509. - DOI - PubMed
-
- Rennert R.C., Sorkin M., Januszyk M., Duscher D., Kosaraju R., Chung M.T., Lennon J., Radiya-Dixit A., Raghvendra S., Maan Z.N., et al. Diabetes impairs the angiogenic potential of adipose-derived stem cells by selectively depleting cellular subpopulations. Stem Cell Res. Ther. 2014;5:79. doi: 10.1186/scrt468. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

