The NLRP3 inflammasome in traumatic brain injury: potential as a biomarker and therapeutic target
- PMID: 32252777
- PMCID: PMC7137518
- DOI: 10.1186/s12974-020-01778-5
The NLRP3 inflammasome in traumatic brain injury: potential as a biomarker and therapeutic target
Abstract
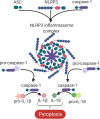
There is a great clinical need to identify the underlying mechanisms, as well as related biomarkers, and treatment targets, for traumatic brain injury (TBI). Neuroinflammation is a central pathophysiological feature of TBI. NLRP3 inflammasome activity is a necessary component of the innate immune response to tissue damage, and dysregulated inflammasome activity has been implicated in a number of neurological conditions. This paper introduces the NLRP3 inflammasome and its implication in the pathogenesis of neuroinflammatory-related conditions, with a particular focus on TBI. Although its role in TBI has only recently been identified, findings suggest that priming and activation of the NLRP3 inflammasome are upregulated following TBI. Moreover, recent studies utilizing specific NLRP3 inhibitors have provided further evidence that this inflammasome is a major driver of neuroinflammation and neurobehavioral disturbances following TBI. In addition, there is emerging evidence that circulating inflammasome-associated proteins may have utility as diagnostic biomarkers of neuroinflammatory conditions, including TBI. Finally, novel and promising areas of research will be highlighted, including the potential involvement of the NLRP3 inflammasome in mild TBI, how factors such as biological sex may affect NLRP3 activity in TBI, and the use of emerging biomarker platforms. Taken together, this review highlights the exciting potential of the NLRP3 inflammasome as a target for treatments and biomarkers that may ultimately be used to improve TBI management.
Keywords: Caspase-1; Chronic traumatic encephalopathy; Concussion; Cytokine; IL-18; IL-1β; Microglia; Mild traumatic brain injury; Neuroinflammation; TBI.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Similar articles
-
NLRP3 inflammasome in traumatic brain injury: Its implication in the disease pathophysiology and potential as a therapeutic target.Life Sci. 2023 Feb 1;314:121352. doi: 10.1016/j.lfs.2022.121352. Epub 2022 Dec 30. Life Sci. 2023. PMID: 36592789 Review.
-
The Role of NLRP3 Inflammasome in the Pathogenesis of Traumatic Brain Injury.Int J Mol Sci. 2020 Aug 27;21(17):6204. doi: 10.3390/ijms21176204. Int J Mol Sci. 2020. PMID: 32867310 Free PMC article. Review.
-
A novel small molecular NLRP3 inflammasome inhibitor alleviates neuroinflammatory response following traumatic brain injury.J Neuroinflammation. 2019 Apr 11;16(1):81. doi: 10.1186/s12974-019-1471-y. J Neuroinflammation. 2019. PMID: 30975164 Free PMC article.
-
Combination therapies and other therapeutic approaches targeting the NLRP3 inflammasome and neuroinflammatory pathways: a promising approach for traumatic brain injury.Immunopharmacol Immunotoxicol. 2025 Apr;47(2):159-175. doi: 10.1080/08923973.2024.2444956. Epub 2025 Jan 6. Immunopharmacol Immunotoxicol. 2025. PMID: 39762721 Review.
-
HIV-1 Tat Primes and Activates Microglial NLRP3 Inflammasome-Mediated Neuroinflammation.J Neurosci. 2017 Mar 29;37(13):3599-3609. doi: 10.1523/JNEUROSCI.3045-16.2017. Epub 2017 Mar 7. J Neurosci. 2017. PMID: 28270571 Free PMC article.
Cited by
-
Brain-specific loss of Abcg1 disturbs cholesterol metabolism and aggravates pyroptosis and neurological deficits after traumatic brain injury.Brain Pathol. 2023 May;33(3):e13126. doi: 10.1111/bpa.13126. Epub 2022 Oct 21. Brain Pathol. 2023. PMID: 36271611 Free PMC article.
-
Extracellular vesicles from hiPSC-NSCs can prevent peripheral inflammation-induced cognitive dysfunction with inflammasome inhibition and improved neurogenesis in the hippocampus.J Neuroinflammation. 2023 Dec 12;20(1):297. doi: 10.1186/s12974-023-02971-y. J Neuroinflammation. 2023. PMID: 38087314 Free PMC article.
-
Understanding microglial responses in large animal models of traumatic brain injury: an underutilized resource for preclinical and translational research.J Neuroinflammation. 2023 Mar 9;20(1):67. doi: 10.1186/s12974-023-02730-z. J Neuroinflammation. 2023. PMID: 36894951 Free PMC article. Review.
-
Repeated closed-head mild traumatic brain injury-induced inflammation is associated with nociceptive sensitization.J Neuroinflammation. 2023 Aug 27;20(1):196. doi: 10.1186/s12974-023-02871-1. J Neuroinflammation. 2023. PMID: 37635235 Free PMC article.
-
A single intranasal dose of human mesenchymal stem cell-derived extracellular vesicles after traumatic brain injury eases neurogenesis decline, synapse loss, and BDNF-ERK-CREB signaling.Front Mol Neurosci. 2023 May 22;16:1185883. doi: 10.3389/fnmol.2023.1185883. eCollection 2023. Front Mol Neurosci. 2023. PMID: 37284464 Free PMC article.
References
-
- McKee AC, Cantu RC, Nowinski CJ, Hedley-Whyte ET, Gavett BE, Budson AE, Santini VE, Lee HS, Kubilus CA, Stern RA. Chronic traumatic encephalopathy in athletes: progressive tauopathy after repetitive head injury. J Neuropathol Exp Neurol. 2009;68:709–735. doi: 10.1097/NEN.0b013e3181a9d503. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

