Cylindromatosis Is Required for Survival of a Subset of Melanoma Cells
- PMID: 32252875
- PMCID: PMC7851542
- DOI: 10.3727/096504020X15861709922491
Cylindromatosis Is Required for Survival of a Subset of Melanoma Cells
Abstract
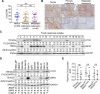
The deubiquitinase cylindromatosis (CYLD) functions as a tumor suppressor inhibiting cell proliferation in many cancer types including melanoma. Here we present evidence that a proportion of melanoma cells are nonetheless addicted to CYLD for survival. The expression levels of CYLD varied widely in melanoma cell lines and melanomas in vivo, with a subset of melanoma cell lines and melanomas displaying even higher levels of CYLD than melanocyte lines and nevi, respectively. Strikingly, although short hairpin RNA (shRNA) knockdown of CYLD promoted, as anticipated, cell proliferation in some melanoma cell lines, it reduced cell viability in a fraction of melanoma cell lines with relatively high levels of CYLD expression and did not impinge on survival and proliferation in a third type of melanoma cell lines. The decrease in cell viability caused by CYLD knockdown was due to induction of apoptosis, as it was associated with activation of the caspase cascade and was abolished by treatment with a general caspase inhibitor. Mechanistic investigations demonstrated that induction of apoptosis by CYLD knockdown was caused by upregulation of receptor-interacting protein kinase 1 (RIPK1) that was associated with elevated K63-linked polyubiquitination of the protein, indicating that CYLD is critical for controlling RIPK1 expression in these cells. Of note, microRNA (miR) profiling showed that miR-99b-3p that was predicted to target the 3-untranslated region (3-UTR) of the CYLD mRNA was reduced in melanoma cell lines with high levels of CYLD compared with melanocyte lines. Further functional studies confirmed that the reduction in miR-99b-3p expression was responsible for the increased expression of CYLD in a highly cell line-specific manner. Taken together, these results reveal an unexpected role of CYLD in promoting survival of a subset of melanoma cells and uncover the heterogeneity of CYLD expression and its biological significance in melanoma.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures





References
-
- Luke JJ, Flaherty KT, Ribas A, Long GV. Targeted agents and immunotherapies: Optimizing outcomes in melanoma. Nat Rev Clin Oncol. 2017;14(8):463–82. - PubMed
-
- Gide TN, Quek C, Menzies AM, Tasker AT, Shang P, Holst J, Madore J, Lim SY, Velickovic R, Wongchenko M, Yan Y, Lo S, Carlino MS, Guminski A, Saw RPM, Pang A, McGuire HM, Palendira U, Thompson JF, Rizos H, Silva IPD, Batten M, Scolyer RA, Long GV, Wilmott JS. Distinct immune cell populations define response to anti-PD-1 monotherapy and anti-PD-1/anti-CTLA-4 combined therapy. Cancer Cell 2019;35(2):238–55.e6. - PubMed
-
- Franke V, Berger DMS, Klop WMC, van der Hiel B, van de Wiel BA, Ter Meulen S, Wouters M, van Houdt WJ, van Akkooi ACJ. High response rates for T-VEC in early metastatic melanoma (stage IIIB/C-IVM1a). Int J Cancer 2019;145(4):974–8. - PubMed
-
- Menzies AM, Long GV. Recent developments in melanoma therapy. JAMA Oncol. 2016;2(10):1259–60. - PubMed
-
- Kozar I, Margue C, Rothengatter S, Haan C, Kreis S. Many ways to resistance: How melanoma cells evade targeted therapies. Biochim Biophys Acta Rev Cancer 2019;1871(2):313–22. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
