Imipenem Population Pharmacokinetics: Therapeutic Drug Monitoring Data Collected in Critically Ill Patients with or without Extracorporeal Membrane Oxygenation
- PMID: 32253220
- PMCID: PMC7269497
- DOI: 10.1128/AAC.00385-20
Imipenem Population Pharmacokinetics: Therapeutic Drug Monitoring Data Collected in Critically Ill Patients with or without Extracorporeal Membrane Oxygenation
Abstract
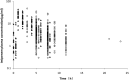
Carbapenem pharmacokinetic (PK) profiles are significantly different in critically ill patients because of the drastic variability of the patients' physiological parameters. Published population PK studies have mainly focused on specific diseases, and the majority of these studies had small sample sizes. The aim of this study was to develop a population PK model of imipenem in critically ill patients that estimated the influence of various clinical and biological covariates and the use of extracorporeal membrane oxygenation (ECMO) and continuous renal replacement therapy (CRRT). A two-compartment population PK model with creatinine clearance (CLCR), body weight (WT), and ECMO as fixed effects was developed using the nonlinear mixed-effects model (NONMEM). A Monte Carlo simulation was performed to evaluate various dosing schemes and different levels of covariates based on the pharmacokinetic/pharmacodynamic index (ƒ%T>MIC) for the range of clinically relevant MICs. The results showed that there may be insufficient drug use in the clinical routine drug dose regimen, and 750 mg every 6 h (q6h) could achieve a higher treatment success rate. The blood concentrations of imipenem in ECMO patients were lower than those in non-ECMO patients; therefore, dosages may need to be increased. The dosage may need adjustment for patients with a CLCR of ≤70 ml/min, but the dose should be lowered carefully to avoid the insufficient drug exposure. Dose adjustment is not necessary for patients with WT ranging from 50 to 80 kg. Due to the large variation in PK profile of imipenem in critically ill patients, therapeutic drug monitoring (TDM) should be carried out to optimize drug regimens.
Keywords: ECMO; critically ill; imipenem; pharmacokinetics/pharmacodynamics; therapeutic drug monitoring.
Copyright © 2020 American Society for Microbiology.
Figures



References
-
- Wu LJ, Jin HJ, Xu LP. 2014. Meta-analysis on meropenem and imipenem/cilastatin sodium in the treatment of bacterial infection. World Notes Antibiotics 35:28–31.
-
- China Medical Education Association, Infection Disease Committee. 2018. Expert consensus on clinical application of pharmacokinetic/pharmacodynamic theory of antibacterial drugs. Chin J Tuberc Respir Dis 41:409–446.
-
- Lipš M, Siller M, Strojil J, Urbánek K, Balík M, Suchánková H. 2014. Pharmacokinetics of imipenem in critically ill patients during empirical treatment of nosocomial pneumonia: a comparison of 0.5-h and 3-h infusions. Int J Antimicrob Agents 44:358–362. doi:10.1016/j.ijantimicag.2014.05.011. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

