Effect of State Immunization Information System Centralized Reminder and Recall on HPV Vaccination Rates
- PMID: 32253263
- PMCID: PMC7193977
- DOI: 10.1542/peds.2019-2689
Effect of State Immunization Information System Centralized Reminder and Recall on HPV Vaccination Rates
Abstract
Background: Although autodialer centralized reminder and recall (C-R/R) from state immunization information systems (IISs) has been shown to raise childhood vaccination rates, its impact on human papillomavirus (HPV) vaccination rates is unclear.
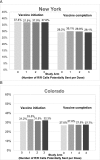
Methods: In a 4-arm pragmatic randomized controlled trial across 2 states, we randomly selected practices representative of the specialty (pediatrics, family medicine, and health center) where children received care. Within each practice, patients 11 to 17.9 years old who had not completed their HPV vaccine series (NY: N = 30 616 in 123 practices; CO: N = 31 502 in 80 practices) were randomly assigned to receive 0, 1, 2, or 3 IIS C-R/R autodialer messages per vaccine dose. We assessed HPV vaccine receipt via the IIS, calculated intervention costs, and compared HPV vaccine series initiation and completion rates across study arms.
Results: In New York, HPV vaccine initiation rates ranged from 37.0% to 37.4%, and completion rates were between 29.1% and 30.1%, with no significant differences across study arms. In Colorado, HPV vaccine initiation rates ranged from 31.2% to 33.5% and were slightly higher for 1 reminder compared with none, but vaccine completion rates, ranging from 27.0% to 27.8%, were similar. On adjusted analyses in Colorado, vaccine initiation rates were slightly higher for 1 and 3 C-R/R messages (adjusted risk ratios 1.07 and 1.04, respectively); completion rates were slightly higher for 1 and 3 C-R/R messages (adjusted risk ratios 1.02 and 1.03, respectively).
Conclusions: IIS-based C-R/R for HPV vaccination did not improve HPV vaccination rates in New York and increased vaccination rates slightly in Colorado.
Copyright © 2020 by the American Academy of Pediatrics.
Conflict of interest statement
POTENTIAL CONFLICT OF INTEREST: The authors have indicated they have no potential conflicts of interest to disclose.
Figures


Comment in
-
Optimizing Human Papillomavirus Immunization: The Role of Centralized Reminder and Recall Systems.Pediatrics. 2020 May;145(5):e20193596. doi: 10.1542/peds.2019-3596. Epub 2020 Apr 6. Pediatrics. 2020. PMID: 32253262 No abstract available.
References
-
- Dunne EF, Unger ER, Sternberg M, et al. Prevalence of HPV infection among females in the United States. JAMA. 2007;297(8):813–819 - PubMed
-
- Zandberg DP, Bhargava R, Badin S, Cullen KJ. The role of human papillomavirus in nongenital cancers. CA Cancer J Clin. 2013;63(1):57–81 - PubMed
-
- Centers for Disease Control and Prevention (CDC) Human papillomavirus-associated cancers - United States, 2004-2008. MMWR Morb Mortal Wkly Rep. 2012;61:258–261 - PubMed

