Redundant and specific roles of cohesin STAG subunits in chromatin looping and transcriptional control
- PMID: 32253279
- PMCID: PMC7197483
- DOI: 10.1101/gr.253211.119
Redundant and specific roles of cohesin STAG subunits in chromatin looping and transcriptional control
Abstract
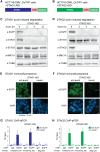
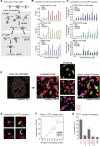
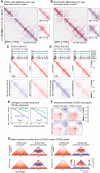
Cohesin is a ring-shaped multiprotein complex that is crucial for 3D genome organization and transcriptional regulation during differentiation and development. It also confers sister chromatid cohesion and facilitates DNA damage repair. Besides its core subunits SMC3, SMC1A, and RAD21, cohesin in somatic cells contains one of two orthologous STAG subunits, STAG1 or STAG2. How these variable subunits affect the function of the cohesin complex is still unclear. STAG1- and STAG2-cohesin were initially proposed to organize cohesion at telomeres and centromeres, respectively. Here, we uncover redundant and specific roles of STAG1 and STAG2 in gene regulation and chromatin looping using HCT116 cells with an auxin-inducible degron (AID) tag fused to either STAG1 or STAG2. Following rapid depletion of either subunit, we perform high-resolution Hi-C, gene expression, and sequential ChIP studies to show that STAG1 and STAG2 do not co-occupy individual binding sites and have distinct ways by which they affect looping and gene expression. These findings are further supported by single-molecule localizations via direct stochastic optical reconstruction microscopy (dSTORM) super-resolution imaging. Since somatic and congenital mutations of the STAG subunits are associated with cancer (STAG2) and intellectual disability syndromes with congenital abnormalities (STAG1 and STAG2), we verified STAG1-/STAG2-dependencies using human neural stem cells, hence highlighting their importance in particular disease contexts.
© 2020 Casa et al.; Published by Cold Spring Harbor Laboratory Press.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
