A dual effect of ursolic acid to the treatment of multiple sclerosis through both immunomodulation and direct remyelination
- PMID: 32253301
- PMCID: PMC7183235
- DOI: 10.1073/pnas.2000208117
A dual effect of ursolic acid to the treatment of multiple sclerosis through both immunomodulation and direct remyelination
Abstract
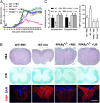
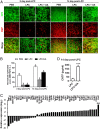
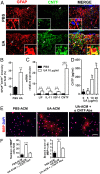
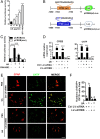
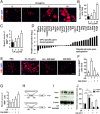
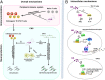
Current multiple sclerosis (MS) medications are mainly immunomodulatory, having little or no effect on neuroregeneration of damaged central nervous system (CNS) tissue; they are thus primarily effective at the acute stage of disease, but much less so at the chronic stage. An MS therapy that has both immunomodulatory and neuroregenerative effects would be highly beneficial. Using multiple in vivo and in vitro strategies, in the present study we demonstrate that ursolic acid (UA), an antiinflammatory natural triterpenoid, also directly promotes oligodendrocyte maturation and CNS myelin repair. Oral treatment with UA significantly decreased disease severity and CNS inflammation and demyelination in experimental autoimmune encephalomyelitis (EAE), an animal model of MS. Importantly, remyelination and neural repair in the CNS were observed even after UA treatment was started on day 60 post immunization when EAE mice had full-blown demyelination and axonal damage. UA treatment also enhanced remyelination in a cuprizone-induced demyelination model in vivo and brain organotypic slice cultures ex vivo and promoted oligodendrocyte maturation in vitro, indicating a direct myelinating capacity. Mechanistically, UA induced promyelinating neurotrophic factor CNTF in astrocytes by peroxisome proliferator-activated receptor γ(PPARγ)/CREB signaling, as well as by up-regulation of myelin-related gene expression during oligodendrocyte maturation via PPARγ activation. Together, our findings demonstrate that UA has significant potential as an oral antiinflammatory and neural repair agent for MS, especially at the chronic-progressive stage.
Keywords: immunomodulation; multiple sclerosis; neural repair; ursolic acid.
Conflict of interest statement
The authors declare no competing interest.
Figures



References
-
- Tintore M., Vidal-Jordana A., Sastre-Garriga J., Treatment of multiple sclerosis: Success from bench to bedside. Nat. Rev. Neurol. 15, 53–58 (2019). - PubMed
-
- Correale J., Gaitán M. I., Ysrraelit M. C., Fiol M. P., Progressive multiple sclerosis: From pathogenic mechanisms to treatment. Brain 140, 527–546 (2017). - PubMed
-
- Stangel M., Kuhlmann T., Matthews P. M., Kilpatrick T. J., Achievements and obstacles of remyelinating therapies in multiple sclerosis. Nat. Rev. Neurol. 13, 742–754 (2017). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

