Effectiveness of convalescent plasma therapy in severe COVID-19 patients
- PMID: 32253318
- PMCID: PMC7196837
- DOI: 10.1073/pnas.2004168117
Effectiveness of convalescent plasma therapy in severe COVID-19 patients
Abstract
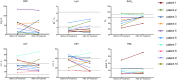
Currently, there are no approved specific antiviral agents for novel coronavirus disease 2019 (COVID-19). In this study, 10 severe patients confirmed by real-time viral RNA test were enrolled prospectively. One dose of 200 mL of convalescent plasma (CP) derived from recently recovered donors with the neutralizing antibody titers above 1:640 was transfused to the patients as an addition to maximal supportive care and antiviral agents. The primary endpoint was the safety of CP transfusion. The second endpoints were the improvement of clinical symptoms and laboratory parameters within 3 d after CP transfusion. The median time from onset of illness to CP transfusion was 16.5 d. After CP transfusion, the level of neutralizing antibody increased rapidly up to 1:640 in five cases, while that of the other four cases maintained at a high level (1:640). The clinical symptoms were significantly improved along with increase of oxyhemoglobin saturation within 3 d. Several parameters tended to improve as compared to pretransfusion, including increased lymphocyte counts (0.65 × 109/L vs. 0.76 × 109/L) and decreased C-reactive protein (55.98 mg/L vs. 18.13 mg/L). Radiological examinations showed varying degrees of absorption of lung lesions within 7 d. The viral load was undetectable after transfusion in seven patients who had previous viremia. No severe adverse effects were observed. This study showed CP therapy was well tolerated and could potentially improve the clinical outcomes through neutralizing viremia in severe COVID-19 cases. The optimal dose and time point, as well as the clinical benefit of CP therapy, needs further investigation in larger well-controlled trials.
Keywords: COVID-19; convalescent plasma; pilot project; treatment outcome.
Copyright © 2020 the Author(s). Published by PNAS.
Conflict of interest statement
The authors declare no competing interest.
Figures


Comment in
-
Get rid of the bad first: Therapeutic plasma exchange with convalescent plasma for severe COVID-19.Proc Natl Acad Sci U S A. 2020 Jun 9;117(23):12526-12527. doi: 10.1073/pnas.2006691117. Epub 2020 May 12. Proc Natl Acad Sci U S A. 2020. PMID: 32398378 Free PMC article. No abstract available.
-
Convalescent plasma for patients with COVID-19.Proc Natl Acad Sci U S A. 2020 Jun 9;117(23):12528. doi: 10.1073/pnas.2006961117. Epub 2020 May 12. Proc Natl Acad Sci U S A. 2020. PMID: 32398379 Free PMC article. No abstract available.
-
COVID-19: are neutralizing antibodies neutralizing enough?Transfusion. 2020 Jul;60(7):1602-1603. doi: 10.1111/trf.15897. Epub 2020 Jun 3. Transfusion. 2020. PMID: 32449171 Free PMC article. No abstract available.
References
-
- World Health Organization , Coronavirus disease (COVID-19) Pandemic. https://www.who.int/emergencies/diseases/novel-coronavirus-2019. Accessed 11 March 2020.
-
- Lu H., Drug treatment options for the 2019-new coronavirus (2019-nCoV). Biosci. Trends 14, 69–71 (2020). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

